Autotransfusion Devices Market: Introduction
Autotransfusion devices are medical instruments used to collect, process, and return a patient's own blood during or after a surgical procedure. This process, known as autotransfusion or cell salvage, helps to minimize the need for allogeneic blood transfusions (blood from a donor) and reduce the risk of transfusion-related complications. The main uses and benefits of autotransfusion devices include:
1. Reduced reliance on donor blood: Autotransfusion reduces the need for allogeneic blood transfusions, which can help address blood shortages and decrease the demand on blood banks.2. Lower risk of transfusion-related complications: Using a patient's own blood minimizes the risk of transfusion reactions, such as allergic reactions, haemolytic reactions, and transfusion-related acute lung injury (TRALI), as well as the transmission of blood-borne infections.
3. Improved patient outcomes: Autotransfusion can help to maintain optimal blood volume during surgery, reduce the risk of postoperative anaemia, and enhance overall patient outcomes.
4. Cost-effectiveness: By reducing the need for donor blood, autotransfusion can lead to cost savings for healthcare facilities and may help to lower the overall cost of surgical procedures.
5. Applicability in various surgeries: Autotransfusion devices are used in a wide range of surgical procedures where significant blood loss is expected, such as orthopaedic, cardiovascular, trauma, and transplant surgeries.
6. Preservation of blood components: The processing of blood through autotransfusion devices helps to maintain the integrity of blood components, such as red blood cells, platelets, and clotting factors, which can contribute to better patient outcomes.
In summary, autotransfusion devices are medical instruments that collect, process, and return a patient's own blood during or after surgery. Their use provides several benefits, including reduced reliance on donor blood, lower risk of transfusion-related complications, improved patient outcomes, cost-effectiveness, and applicability in various surgical procedures.
Autotransfusion Devices Market Segmentations
The market can be categorised into product type, application, end user, and region.Market Breakup by Product type
- Autotransfusion System
2. Post-Operative Autotransfusion System
3. Dual-Mode Autotransfusion Systems
- Consumables and Accessories
Market Breakup by Application
- Cardiovascular Surgeries
- Orthopedic Surgeries
- Neurological Surgeries
- Organ Transplantation
- Obstetrics and Gynecological Surgeries
- Others
Market Breakup by End user
- Hospitals
- Specialty Clinics
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Autotransfusion Devices Market Scenario
The global autotransfusion devices market is driven by the increasing demand for medical instruments that reduce the reliance on allogeneic blood transfusions and enhance patient outcomes during surgical procedures. These devices are used in various surgeries, such as orthopaedic, cardiovascular, trauma, and transplant surgeries, where significant blood loss is expected. The market encompasses the development, production, distribution, and sales of autotransfusion devices, as well as research and development of new and improved technologies.Market Drivers
Key factors propelling the autotransfusion devices market include the rising number of surgical procedures worldwide, technological advancements in medical devices, and the growing awareness of the benefits of autotransfusion. Additionally, the market is driven by the increasing prevalence of chronic diseases that may necessitate surgical intervention, a rising global aging population, and the need to address blood shortages in healthcare facilities.Market Challenges
Despite the growing demand for autotransfusion devices, the market faces some challenges. High costs associated with these devices and the requirement for skilled professionals to operate them may limit their adoption, particularly in low- and middle-income countries where healthcare resources are constrained. Furthermore, the lack of awareness regarding autotransfusion devices in some regions may hinder market growth.Regional Analysis
Geographically, the global autotransfusion devices market can be segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America dominates the market due to its advanced healthcare infrastructure, increased adoption of advanced medical devices, and the presence of key market players. Europe follows closely, while Asia-Pacific is anticipated to be the fastest-growing region due to the increasing prevalence of chronic diseases, improving healthcare infrastructure, and rising healthcare expenditures in the region.Future Outlook
The autotransfusion devices market is expected to continue growing in the coming years, primarily driven by the increasing number of surgical procedures, technological advancements, and the need to reduce reliance on allogeneic blood transfusions. As new technologies emerge and healthcare systems continue to evolve, the market may experience increased access to autotransfusion devices, leading to improved surgical outcomes and enhanced patient care worldwide.Key Players in the Global Autotransfusion Devices Market
The report gives an in-depth analysis of the key players involved in the autotransfusion devices market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- LivaNova PLC
- Haemonetics Corporation
- Medtronic plc
- Fresenius SE & Co. KGaA
- Becton, Dickinson, and Company (BD)
- Redax
- Haemonetics Corporation
- Braile Biomedica
- Gen world Medical Devices
- SARSTEDT AG & CO. Kg
- Teleflex Incorporated
- Zimmer Biomet Holdings Inc.
Table of Contents
Companies Mentioned
- Livanova plc
- Haemonetics Corporation
- Medtronic plc
- Fresenius Se & Co. Kgaa
- Becton, Dickinson, and Company (Bd)
- Redax
- Haemonetics Corporation
- Braile Biomedica
- Gen World Medical Devices
- Sarstedt AG & Co. Kg
- Teleflex Incorporated
- Zimmer Biomet Holdings Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
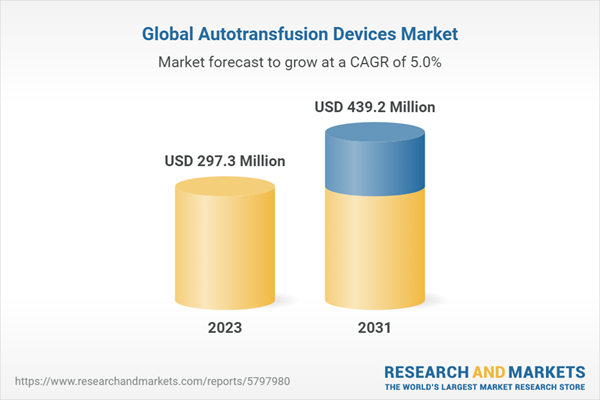
| Estimated Market Value ( USD | $ 297.3 Million |
| Forecasted Market Value ( USD | $ 439.2 Million |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |