Sleep Apnea Device Market: Introduction
A sleep apnea device is a medical apparatus designed to treat sleep apnea, a disorder characterized by repeated pauses in breathing or shallow breaths during sleep. These pauses, known as apneas or hypopneas, disrupt normal sleep patterns and can lead to poor sleep quality, daytime fatigue, and long-term health complications if left untreated. The most common type of sleep apnea device is the Continuous Positive Airway Pressure (CPAP) machine, which delivers a steady stream of air through a mask worn over the nose and/or mouth, keeping the airway open and preventing the collapse of soft tissues that cause breathing disruptions.Other types of sleep apnea devices include Bi-level Positive Airway Pressure (BiPAP) machines, which provide varying levels of pressure for inhalation and exhalation, and Adaptive Servo-Ventilation (ASV) devices, which adjust airflow based on the user's breathing patterns. Additionally, oral appliances, such as mandibular advancement devices (MADs) and tongue retaining devices (TRDs), can be used to reposition the lower jaw or tongue, helping to maintain an open airway during sleep.
Sleep apnea devices are primarily used to improve sleep quality, reduce daytime fatigue, and prevent long-term health complications associated with untreated sleep apnea, such as hypertension, heart disease, stroke, and diabetes. By ensuring continuous airflow and maintaining open airways, these devices aim to minimize the occurrence of apneas and hypopneas, resulting in a more restful and healthier sleep.
Sleep Apnea Device Market Segmentations
The market can be categorised into diagnosis method, therapeutic devices, end user, and major region.Market Breakup by Diagnosis Method
- Polysomnography Devices (PSG)
- Ambulatory PSG Devices
- Clinical PSG Devices
- Oximeters
- Fingertip Oximeters
- Handheld Oximeters
- Wrist-worn Oximeter
- Tabletop Oximeter
- Home Sleep Testing Devices
- Actigraphy Devices
- Others
Market Breakup by Therapeutic Devices
- Oxygen Devices
- Oxygen Concentrators
- Portable Oxygen Concentrators
- Liquid Portable Oxygen
- Oral Appliances Therapeutic Devices
- Adaptive Servo Ventilation (ASV) Devices
- Masks and Accessories
Market Breakup by End User
- Sleep Laboratories and Hospitals
- Clinics
- Homecare Settings/Individuals
- Others
Sleep Apnea Device Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
The sleep apnea device market has been experiencing significant growth due to a variety of factors. The rising prevalence of sleep apnea, coupled with an increasing awareness of its health risks and complications, has led to a greater demand for effective treatment options. Additionally, the aging global population and the growing prevalence of obesity, both of which are risk factors for sleep apnea, contribute to the expansion of the market.
Technological advancements in sleep apnea devices have resulted in more compact, comfortable, and user-friendly designs, which has further fuelled their adoption. Innovations in connectivity and data analysis capabilities have also improved patient monitoring and compliance, making these devices more appealing to both patients and healthcare providers.
Moreover, the expansion of home-based care and telemedicine has driven the demand for sleep apnea devices that can be used in non-clinical settings, allowing patients to manage their condition from the comfort of their homes. Finally, government initiatives, public health campaigns, and favorable reimbursement policies in many countries have played a crucial role in promoting the use of sleep apnea devices and making them more accessible to patients.
In summary, the sleep apnea device market is experiencing growth due to factors such as increased disease prevalence, technological advancements, an aging global population, rising obesity rates, and supportive government policies.
Key Players in the Global Sleep Apnea Device Market
The report gives an in-depth analysis of the key players involved in the sleep apnea device market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Fisher & Paykel Healthcare Limited
- Invacare Corporation
- Natus Medical Incorporated
- Cadwell Industries Inc
- Vyaire Medical, Inc
- Nihon Kohden Corporation
- Koninklijke Philips N.V
- ResMed Corp
- Somnomed
- Compumedics
- Drive Devilbiss Healthcare
- Lowenstein Medical Technology Gmbh
- BMC Medical Co., Ltd.
Table of Contents
Companies Mentioned
- Fisher & Paykel Healthcare Limited
- Invacare Corporation
- Natus Medical Incorporated
- Cadwell Industries Inc.
- Vyaire Medical, Inc.
- Nihon Kohden Corporation
- Koninklijke Philips N.V.
- Resmed Corp.
- Somnomed
- Compumedics
- Drive Devilbiss Healthcare
- Lowenstein Medical Technology GmbH
- Bmc Medical Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
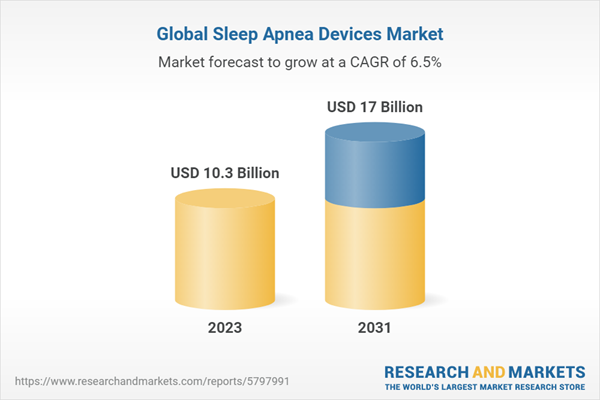
| Estimated Market Value ( USD | $ 10.3 Billion |
| Forecasted Market Value ( USD | $ 17 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |