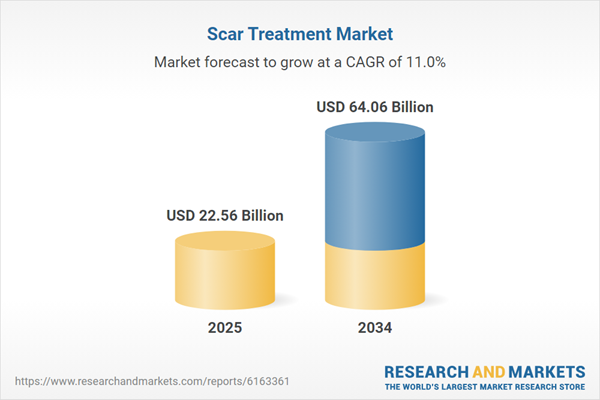
Scar Treatment Market Overview
Scar treatment includes a range of techniques and products used to reduce, mask, or completely remove scars on the skin. Scars may be caused by injuries, surgeries, burns, acne, and other skin disorders. It develops as a result of the body's natural healing process. Topical creams, gels, silicone sheets, laser therapy, surgery, and injections are amongst the common treatments for treating scars.The scar treatment market growth is driven by individuals becoming more self-conscious about their appearance. This has resulted in increased scar removal and reduction treatments. Consequently, resulting in increased incidence in approvals from regulatory authorities. For instance, in August 2023, FDA approved Sofwave Medical Ltd.'s Precise SUPERB Applicator to treat acne scars, expanding its list of approved uses beyond cellulite. After three sessions, a multicenter clinical research spread across multiple US locations showed a notable improvement in the appearance of acne scars. Most patients expressed relief and great pleasure with their outcomes. There were no significant adverse events in the research, which showed a positive safety profile.
To meet the rising scar treatment market demand, mergers and collaborations are witnessing a significant surge. In June 2023, International Medical Lasers (IML) partnered with DEKA Trio to offer laser accessories, including a scanner for CO2 laser-assisted scar reconstruction. The terms of agreement also focused on an FDA approved novel scanner for surgical scar correction By collaborating with global medical providers and manufacturers, new-age and technology driven companies are bringing cutting-edge medical technology to the markets, further driving its growth.
The scar treatment market share for products and services continues to expand, driven by rising application in the realm of medical aesthetics for non-surgical alternatives. People are looking for less intrusive ways to repair scars and rejuvenate their skin instead of undergoing typical surgical procedures. As laser-based procedures for skin rejuvenation and scar correction become more common, there is an increasing demand for cutting-edge instruments and technology that can produce good outcomes with little risk or downtime.
Scar Treatment Market Trends
Scar Treatment Market Segmentation
Market Breakup by Scar Type
- Atrophic Scars
- Hypertrophic and Keloid Scars
- Contracture Scars
- Stretch Marks
Market Breakup by Product
- Topical Products
- Creams
- Gels
- Silicon Sheets
- Others
- Laser Products
- CO2 Laser
- Pulse-dyed Laser
- Others
- Injectable
- Others
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Scar Treatment Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Alliance Pharma PLC
- Perrigo Company plc
- Newmedical Technology Inc.
- Sonoma Pharmaceuticals, Inc.
- HRA Pharma
- Boston Scientific Corporation
- CCA Industries, Inc.
- Avita Medical,Inc.
- Smith & Nephew PLC
- Shanghai Fosun Pharmaceutical(Group)Co., Ltd.
- Sientra, Inc.
- Mölnlycke Health Care AB
- Merz Pharma
- POLYTECH Health & Aesthetics GmbH
- CYNOSURE LUTRONIC
Key Highlights of the Report
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:- Scar Type
- Product
- End User
- Region
- Atrophic Scars
- Hypertrophic and Keloid Scars
- Contracture Scars
- Stretch Marks
- Topical Products
- Laser Products
- Injectable
- Others
- Hospitals
- Ambulatory Surgical Centers
- Others
- United States
- EU-4 and the United Kingdom
- Japan
- India
- Market Drivers and Constraints
- SWOT Analysis
- Porter's Five Forces Model
- Key Demand Indicators
- Key Price Indicators
- Industry Events, Initiatives, and Trends
- Value Chain Analysis
- Market Structure
- Company Profiles
- Financial Analysis
- Product Portfolio
- Demographic Reach and Achievements
- Mergers and Acquisitions
- Certifications
- Alliance Pharma PLC
- Perrigo Company plc
- Newmedical Technology Inc.
- Sonoma Pharmaceuticals, Inc.
- HRA Pharma
- Boston Scientific Corporation
- CCA Industries, Inc.
- Avita Medical,Inc.
- Smith & Nephew PLC
- Shanghai Fosun Pharmaceutical(Group)Co., Ltd.
- Sientra, Inc.
- Mölnlycke Health Care AB
- Merz Pharma
- POLYTECH Health & Aesthetics GmbH
- CYNOSURE LUTRONIC
- How is the market landscape expected to evolve in the forecast period?
- What future trends can be anticipated in the market?
- What will be the effect of each driver, challenge, and opportunity on the market?
- Which regional market is expected to experience expedited growth during the forecast period?
- Which production type will lead the market growth?
- Which end user is expected to dominate the respective market segment?
- What are the key research initiatives expected to boost the market value during the forecast period?
- Which treatment type is poised to make the highest impact on the Scar treatment market growth?
- What are the market shares, strategies, and product portfolios of the leading players in the market?
- How is the regulatory landscape shaping the market?
- What are the market dynamics, trends, and growth prospects in the key regional markets?
- How is technology innovation impacting the development of scar treatment?
- What are the investment opportunities and potential risks associated with the market?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Alliance Pharma PLC
- Perrigo Company plc
- Newmedical Technology Inc.
- Sonoma Pharmaceuticals, Inc.
- HRA Pharma
- Boston Scientific Corporation
- CCA Industries, Inc.
- Avita Medical,Inc.
- Smith & Nephew PLC
- Shanghai Fosun Pharmaceutical?Group?Co., Ltd.
- Sientra, Inc.
- Mölnlycke Health Care AB
- Merz Pharma
- POLYTECH Health & Aesthetics GmbH
- CYNOSURE LUTRONIC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 22.56 Billion |
| Forecasted Market Value ( USD | $ 64.06 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |