U.S. Radiopharmaceuticals Market Size and Growth 2025-2034
The U.S. radiopharmaceuticals market is poised for significant growth, driven by increasing demand for advanced diagnostic imaging and targeted radiotherapy across the region. With continuous innovation and regulatory support, the market is expected to expand steadily, offering new treatment avenues for various cancers and neurological disorders.North America holds a substantial share in the market due to high healthcare expenditure, robust research infrastructure, and strong regulatory backing. The region benefits from well-established nuclear medicine facilities and the presence of leading industry players. The rising adoption of PET and SPECT imaging further accelerates market expansion.
In June 2024, Orano Med inaugurated the world’s first industrial-scale lead-212 radiopharmaceuticals manufacturing facility, ATLab (Alpha Therapy Laboratory), in Brownsburg, in the United States. This USD 20 million investment marks a significant step in addressing radiopharmaceutical production and supply challenges. The facility is expected to manufacture 10,000 doses annually by 2025, with a vision to scale tenfold by 2030.
The Asia-Pacific region is experiencing rapid growth, driven by increasing cancer prevalence, aging populations, and improved healthcare infrastructure. Countries like China, Japan, and India are actively expanding their nuclear medicine capabilities to enhance diagnostic accuracy and therapeutic outcomes.
Additionally, the region's augmented investments in research and development are bolstering radiopharmaceutical innovation. Governments and private enterprises are collaborating to establish new production facilities, ensuring a steady supply of radiopharmaceuticals and promoting advancements in targeted radionuclide therapy.
Radiopharmaceuticals are radioactive compounds used in medical imaging and therapy, primarily for cancer and neurological disorders. They enable precise disease diagnosis through PET and SPECT imaging and offer targeted treatments like radioligand therapy.
The radiopharmaceuticals market is expanding due to the rising demand for precision medicine and targeted therapies. Increased FDA approvals, technological advancements, and a strong pipeline of novel radiopharmaceuticals are expected to fuel growth, making nuclear medicine a critical component of modern healthcare.
Radiopharmaceuticals Market Growth Factors
Investment in radiopharmaceutical manufacturing has surged as companies expand production capabilities. Biotech firms and contract service organizations are funding state-of-the-art facilities. There have been ongoing efforts to boost market growth by increasing scalability, enhancing production efficiency, and accelerating innovation.Advancements in theranostics (combining diagnostic and therapeutic functions), are resulting in real-time treatment adjustments and improved patient outcomes. This boosts market growth by elevating treatment precision and efficiency.
Radiopharmaceuticals are revolutionizing cancer treatment by delivering precise, high-dose radiation directly to tumor cells. A January 2024 review revealed detailed advances in radiolabeled antibodies, peptides, and nucleic acids, emphasizing enhanced targeting and therapeutic efficiency in cancer therapies. These innovations enable more accurate tumor localization and effective eradication of malignant cells, significantly improving patient outcomes and accelerating market growth and dynamics.
Merger and acquisitions are reshaping the radiopharmaceutical landscape. For example, in December 2023, Bristol Myers Squibb acquired RayzeBio for USD 4.1 billion, securing promising radiopharma assets and strengthening its product pipeline. These deals reflect confidence in the future of radiopharmaceutical innovation, which will invite global investment and accelerate market growth.
Clinical trials are pivotal for validating radiopharmaceutical efficacy. For instance, in January 2025, ITM, a German radiopharma company, announced positive phase 3 COMPETE trial results for ITM-11. It is a targeted radionuclide therapeutic which demonstrated superior performance against standard therapies. Such breakthrough drives market confidence and dynamics, accelerating clinical advancements and investment in innovative treatments, substantially promising.
Collaboration and partnerships are enhancing radiopharmaceutical development. One such collaboration happened in July 2024, where Eli Lilly signed a USD 140 million upfront deal with Radionetics. This alliance, along with strategic acquisitions, fosters innovative research and drives technological integration and robust investment, significantly impacting industry growth and dynamics.
Radiopharmaceuticals Market Dynamics
Driver
The radiopharmaceuticals market is rapidly evolving with innovative treatment methods and emerging research. Personalized medicine acts as a key market driver by tailoring therapies to individual patient profiles. This approach not only enhances treatment efficacy but also drives demand for targeted radiopharmaceuticals, positioning the industry for growth and improved clinical outcomes across diverse patient populations.Advances in genomics and molecular imaging contribute to developing highly personalized radiopharmaceuticals. A review published in 2023 indicated how unique molecular profiles enable safe, effective treatments. Additionally, February 2025 research introduced a personalized metronomic radiopharmaceutical therapy paradigm, further solidifying the market’s transformative potential.
Advancements in genomics and molecular imaging have revolutionized precision-targeted therapies, enabling the development of highly individualized treatments. Personalized medicine continues to reshape therapeutic strategies, enhancing precision in radiopharmaceutical applications. Its integration into clinical practice not only improves patient outcomes but also creates a robust market foundation. This evolution encourages ongoing research and investment, ensuring that the sector remains dynamic and responsive to emerging medical needs.
Restraints
The short half-lives of radiopharmaceuticals present a significant market restraint. The rapid decay of these agents’ challenges storage, distribution, and timely administration, potentially limiting widespread clinical adoption. Such limitations necessitate continuous innovation in radiopharmaceutical design and logistics to overcome inherent challenges and meet market demands effectively.Efforts to address the short half-life restraint include developing more stable compounds and advanced delivery systems. These innovations aim to extend effective dosing periods without compromising patient safety. By enhancing formulation stability and optimizing distribution channels, manufacturers can mitigate decay issues, thereby improving therapeutic efficacy and market penetration despite the inherent temporal limitations of radiopharmaceutical agents.
Opportunities
Neurological applications represent a promising market opportunity for radiopharmaceuticals. Advanced imaging techniques and targeted agents facilitate the early diagnosis and effective treatment of neurological disorders. This emerging area drives research investment and clinical trials, as improved brain imaging and therapy offer new avenues for managing conditions such as Alzheimer’s disease and other neurodegenerative disorders.The expansion of neurological applications in radiopharmaceuticals holds considerable promise. Enhanced imaging modalities enable more accurate disease detection and monitoring, while targeted therapies improve patient outcomes. As research continues to unveil novel biomarkers and imaging agents, the market is set to benefit from increased clinical adoption and a growing focus on addressing unmet neurological healthcare needs.
Radioisotopes Insights
Based on radioisotopes, the market is divided into Iodine I, Technetium 99m, Fluorine 18, Gallium 68, Radium 223, and Zirconium 89. Among these, Technetium 99m is expected to have a significant share in the market due to its widespread use in diagnostic imaging, particularly in cardiology and oncology. Its short half-life, high availability, and superior imaging capabilities also contribute to its major market share.Other radioisotopes, including Iodine I, Fluorine 18, Gallium 68, Radium 223, and Zirconium 89, serve diverse diagnostic and therapeutic applications. Fluorine 18 is gaining traction in positron emission tomography (PET) imaging, while Radium 223 is increasingly used in targeted cancer therapies. The growing adoption of these isotopes highlights their expanding role in precision medicine.
Application Insights
The market is segmented by application into cancer (prostate, lung, breast, and others), neurology, cardiology, and others. The cancer segment is expected to hold the largest share due to the rising global cancer burden and increasing adoption of radiopharmaceuticals in oncology. Prostate cancer treatments using radioligand therapies, such as Lutetium-177 PSMA, are driving demand.Neurology is anticipated to witness significant growth, driven by the increasing prevalence of neurodegenerative disorders like Alzheimer’s and Parkinson’s. Radiopharmaceuticals play a crucial role in early diagnosis and disease monitoring, leading to greater adoption in this segment.
Cardiology, along with other applications, continues to contribute significantly to the market. Nuclear imaging techniques, such as myocardial perfusion imaging using Technetium 99m, remain essential in detecting cardiovascular diseases. Emerging applications in inflammatory and infectious disease imaging further expand the market landscape.
Type Insights
The market is segmented into diagnostic and therapeutic radiopharmaceuticals. Diagnostic radiopharmaceuticals are likely to have a substantial market share, primarily due to the extensive use of SPECT (single photon emission computed tomography) and PET (positron emission tomography) imaging in disease detection. The growing preference for non-invasive diagnostic techniques and advancements in imaging technologies further support their strong market position.The growth of diagnostic radiopharmaceuticals is reinforced by increasing demand for early disease detection. PET imaging, particularly with Fluorine 18 and Gallium 68, is widely used in oncology and neurology, ensuring sustained market growth. Continuous research and development efforts enhance the efficiency and accuracy of diagnostic radiopharmaceuticals.
Therapeutic radiopharmaceuticals are gaining momentum, particularly in oncology. Lutetium 177 and Actinium 225-based therapies are revolutionizing targeted cancer treatment. The shift towards personalized medicine and radioligand therapies is accelerating the demand for therapeutic applications, driving innovation and market expansion.
End User Insights
Based on the end user, the market is segmented into hospitals, diagnostic imaging centers, research and academic institutes, among others. Hospitals are expected to hold the largest share due to their role in advanced radiopharmaceutical therapies and imaging procedures. The integration of nuclear medicine into hospital-based oncology and cardiology departments supports this dominance.The widespread adoption of radiopharmaceuticals in hospitals is driven by the availability of specialized facilities, trained personnel, and regulatory compliance. As more hospitals establish nuclear medicine departments, accessibility to radiopharmaceutical treatments continues to improve.
Diagnostic imaging centers are expected to experience rapid growth due to the rising demand for outpatient diagnostic procedures. The convenience, cost-effectiveness, and increasing accessibility of nuclear imaging services contribute to the expansion of this segment.
Research and academic institutes, along with other end users, also play a vital role in the market. Ongoing research in nuclear medicine, clinical trials for novel radiopharmaceuticals, and collaborations with pharmaceutical companies are driving innovation and market evolution.
Radiopharmaceuticals Market Companies
Key players in the market drive innovation through collaborations, acquisitions, and research, enhancing diagnostic imaging and expanding global access to advanced molecular imaging solutions. Some major players include:Bayer AG
Bayer AG, founded in 1863 and headquartered in Leverkusen, Germany, is a global pharmaceutical leader. In the radiopharmaceuticals market, Bayer is pioneering targeted radionuclide therapies. In May 2024, it launched a Phase I trial for 225Ac-PSMA-Trillium, a next-generation alpha therapy for metastatic castration-resistant prostate cancer, reinforcing its commitment to precision oncology.Jubilant Pharmova Limited
Jubilant Pharmova Limited, an Indian company, was established in 1978. It plays a significant role in the radiopharmaceuticals market through Jubilant Radiopharma, its U.S.-based business segment. With an extensive radiopharmacy network, it enhances nuclear medicine services, offering FDA-approved radiopharmaceuticals and diagnostic solutions. In October 2024, it partnered with Simplified Imaging Solutions to launch the first comprehensive nuclear medicine solution in the U.S., streamlining lab operations and improving efficiency.Novartis AG
Founded in 1996 and headquartered in Basel, Switzerland, Novartis AG is a global pharmaceutical leader. It pioneered radioligand therapy (RLT), transforming cancer care. In March 2025, the FDA approved Pluvicto, an intravenous radioligand therapy for earlier use in prostate-specific membrane antigen (PSMA)-positive metastatic prostate cancer. This underscores Novartis’s commitment to advancing precision oncology and expanding treatment options for patients worldwide.General Electric Company
General Electric Company, headquartered in Boston, Massachusetts, is a global leader in healthcare technology. In March 2025, GE HealthCare acquired full ownership of Nihon Medi-Physics, a leading radiopharmaceutical company in Japan. This acquisition strengthens its position in molecular imaging, enhancing diagnostic capabilities in neurology, cardiology, and oncology.Other players in the market are: Iso-Tex Diagnostics, Inc., Lantheus Holdings, Inc., Eli Lilly and Company, Siemens AG, Curium Pharma and Cardinal Health Inc
Recent Developments
- In March 2025, India’s Department of Atomic Energy (DAE) launched new radiopharmaceuticals to enhance cancer treatment. Bhabha Atomic Research Centre (BARC) indigenized key radioisotopes, ensuring affordability. These advancements bolster the radiopharmaceuticals market, improving cancer diagnostics and therapies while reducing dependency on imports.
- In January 2025, Lantheus announced a USD 250 million acquisition of Evergreen Theragnostics, a clinical-stage radiopharmaceutical CDMO. The deal grants Lantheus access to scalable radioligand therapy manufacturing and Octevy, a PET diagnostic for neuroendocrine tumors, reinforcing its position in the expanding radiopharmaceuticals market.
- In January 2025, Altamira Therapeutics partnered with a radiopharmaceutical firm to evaluate its RNA nanoparticle delivery platform for cancer treatment. This collaboration is aimed at enhancing tumor targeting, potentially leading to a licensing and supply agreement, reflecting innovation in the expanding radiopharmaceuticals market.
- In June 2024, ITM Isotope Technologies Munich SE secured EUR 188 million to advance its radiopharmaceutical pipeline. The investment will accelerate the potential market launch of ITM-11 for gastroenteropancreatic neuroendocrine tumors and expand production of Lutetium-177 and Actinium-225, strengthening ITM’s leadership in medical radioisotope manufacturing.
- In December 2023, Bristol Myers Squibb acquired RayzeBio for USD 4.1 billion, enhancing its radiopharmaceutical portfolio. RayzeBio’s actinium-based RPTs, including RYZ101 for gastroenteropancreatic neuroendocrine tumors, strengthen Bristol Myers Squibb’s oncology pipeline and manufacturing capabilities, advancing targeted cancer therapies.
Segments Covered in the Radiopharmaceuticals Market Report
Radiopharmaceuticals Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Type Outlook
- Diagnostic Radiopharmaceuticals
- Therapeutic Radiopharmaceuticals
Sources Outlook
- Cyclotron
- Nuclear Reactors
Radioisotopes Outlook
- Iodine I
- Technetium 99m
- Fluorine 18
- Gallium 68
- Radium 223
- Zirconium 89
- Others
Application Outlook
- Cancer
- Prostate
- Lung
- Breast
- Others
- Neurology
- Cardiology
- Others
End User Outlook
- Hospitals
- Diagnostic Imaging Centers
- Research and Academic Institutes
- Others
Region Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
More Insights On
Radiopharmaceutical Theranostics Market
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Bayer AG
- Jubilant Pharmova Limited
- Novartis AG
- General Electric Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
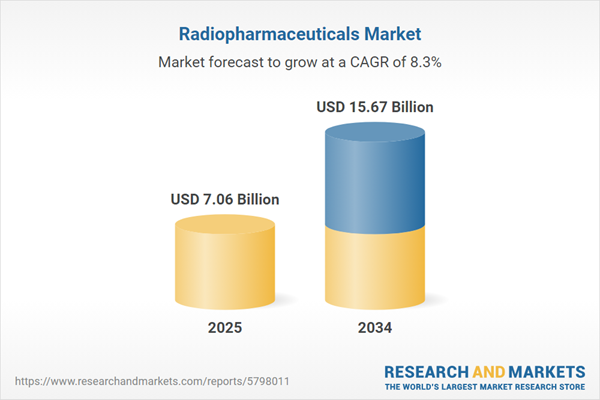
| Estimated Market Value ( USD | $ 7.06 Billion |
| Forecasted Market Value ( USD | $ 15.67 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |