Cranial Implants Market Overview
Cranial implants are medical devices made from materials such as titanium and polyethylene to repair or replace bone defects in the skull resulting from different conditions. They repair the protective framework of the skull using cutting-edge imaging and 3D printing techniques. These implants provide structural support and are crucial in aesthetic restoration which also enhance patients' quality of life by reducing the chances of rejection and infection. Cranial implants are required for conditions such as traumatic brain injuries, congenital skull defects, and tumor removal, as these indications often result in cranial defects. They also address cosmetic abnormalities, improving mental health and social interactions through the restoration of a normal skull shape.The cranial implants are categorized based on materials and personalized design. It includes material options such as titanium for durability, PMMA for easy shaping, and porous polyethylene for adaptability. Customized implants are made from the CT scan information which guarantee precise fit and optimal result. These implants are available in common sizes and shapes with every category offering distinct advantages depending on factors such as defect size and patient health.
Cranial Implants Market Growth Drivers
Increasing Cranial Surgeries Spur Market Growth
The growing demand for cranial implants is being driven by an increase in traumatic brain injuries (TBI), congenital skull defects, and the necessity for tumor resection surgeries. The U.S. Centers for Disease Control and Prevention (CDC) reports that TBI is a leading cause of death in the region. There were approximately 2 lakh TBI-related hospitalizations in 2020 and around 69,473 TBI-related deaths in 2021.Higher rates of road accidents and sports injuries also play a significant role in the increase in cranial surgeries.Technological Advancements Drive the Global Cranial Implants Market Demand
Technological progress in 3D printing, imaging, materials, surface modification, and CAD/CAM technologies are fueling market growth. In April 2024, 3D Systems obtained FDA clearance for the EXT 220 MED printer. It uses medical-grade PEEK materials to create 3D-printed craniums for cranial reconstruction. The printer offers a cleanroom environment and efficient workflow, reducing material waste and costs for hospitals. Additionally, in May 2024, researchers from the USC's Keck School of Medicine and Caltech implanted a transparent cranial window in a patient for high-resolution brain imaging using functional ultrasound imaging (fUSI). This non-invasive method shows promise for patient monitoring and brain function studies. These advancements enhance surgical results, and patient contentment and lower the chances of infection.Cranial Implants Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:
Increased Geriatric Population
Brain injuries and tumors are more common in older individuals, necessitating cranial surgery in many cases. Medical progress and advancements in surgery have enhanced results for elderly patients, leading to an increase in the frequency of cranial surgeries. The increasing focus on improving quality of life and enhancing aesthetics in the elderly is also fueling the need for cranial implants.Use of Advanced Materials
There is increased research into using advanced materials for better performance and outcomes for patients. Material advancements have resulted in implants that possess improved biocompatibility, strength, and integration capabilities. Bioresorbable polymers are gaining popularity as they slowly dissolve and are replaced by natural bone. It decreases the necessity for additional surgeries. Titanium is commonly used with enhanced surface treatments to improve bone integration and decrease infection risks. Advanced polymers such as porous polyethylene offer structural support and durable outcomes with tissue ingrowth, meeting both medical and aesthetic requirements for cranial reconstruction.Rising Research and Development Activities
Increased research and development (R&D) activities for advancements in designs, materials, and manufacturing methods are amongst the major market trends. Cutting-edge technologies such as imaging and computer-aided design allow for custom implants to improve surgical results. Current studies are concentrating on novel materials such as bioresorbable polymers and nanocomposites to enhance biocompatibility.Rising Awareness Regarding Public Health
The rising awareness in public has resulted in a stronger focus on prompt and efficient care for head injuries and abnormalities. Healthcare institutions are advocating for the advantages of cranial implants, urging both patients and medical professionals to explore more advanced surgical choices. Campaigns by healthcare organization also highlight the cranial implant benefits. Government programs promote trauma care and neurosurgery. Additionally, diagnostic equipment assists in early detection and treatment.Cranial Implants Market Segmentation
The report titled “Cranial Implants Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Customized
- Non-Customized
Market Breakup by Material Type
- Polymer
- Ceramic
- Metal
Market Breakup by End User
- Specialty Neurosurgery Centers
- Hospitals
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Cranial Implants Market Share
Market Share Based on Product Type to Undergo Growth
The market is divided into customized and non-customized product types. Out of which customized products are expected to dominate the market due to its growing preference among surgeons. Demand for custom cranial implants is increasing due to the unique characteristics of skull injuries and the rising prevalence of brain tumors and accidents. Manufacturers collaborate with surgeons to enhance market presence and customer base. Skilled practitioners in developed economies are expected to further drive demand for these specialized implants.Cranial Implants Market Analysis by Region
Based on region, the market report covers North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa. North America is expected to dominate the market because of advanced healthcare, technology utilization, and research and development investments. The presence of established manufacturers of medical devices and reimbursement policies in this region also play a major role in market expansion.Europe is also poised to have a significant market share due to its robust healthcare systems and regulatory framework. The cranial implants market in the Asia-Pacific region is expanding quickly because of improved healthcare facilities, heightened awareness, and growing healthcare expenditure. Technological advancements, along with increased incidence of cranial injuries are the major factors influencing the market.
Leading Players in the Cranial Implants Market
The key features of the market report include patent analysis, grant analysis, funding, and investment analysis as well as strategic initiatives including recent partnerships and collaborations by the leading players. The major companies in the market are as follows:Zimmer Biomet Holdings, Inc.
Zimmer Biomet, founded in 1927 and based in Indiana, USA, is a leading medical technology company providing advanced implants and digital technologies for patients. Their products, like the HTR-PEKK Patient Matched Cranial Implant, utilize cutting-edge laser sintering and 3D printing for personalized solutions.Medartis AG
Medartis is a global leader in innovative implants for osteosynthesis in cranio-maxillofacial and extremity surgeries. Their MODUS® Cranium portfolio includes solutions for protecting the brain from injuries, fractures, and tumors. With a focus on optimal results, Medartis offers a wide range of plates for various indications. The company boasts a diverse portfolio, providing surgeons with flexibility and choice.Medtronic Plc.
Medtronic plc is a medical technology company based in Dublin, Ireland, established in 1949. They offer the TiMesh Cranial Plating System, which includes plates, screws, and meshes for neurosurgical procedures such as cranial flap fixation. It also reinforces weak bony tissues in orthopedic surgeries. The system is easy to use and provides a wide selection of options to meet the needs of neurosurgeons and support staff.
Ortho Baltic, UAB
Ortho Baltic specializes in designing and producing patient-specific medical devices for complex medical conditions. It was established in 2021. Their patient-specific cranial implants are plate-type devices are used in cranioplasty to prevent brain injuries and infections, correct deformations, and restore skull aesthetics.Other players in the market Attenborough Medical SRL, B. Braun Melsungen AG, Kelyniam Global Inc., OsteoMed Corporation, OssDsign AB, Skulle Implants Corporation, Stryker Corporation, Tecomet Inc., and Xilloc Medical B.V.
Key Questions Answered in the Cranial Implants Market Report
- What was the global cranial implants market value be in 2024?
- What is the global cranial implants market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on product type?
- What is market segmentation based on material type?
- Who are the major end users of the market?
- What are the major factors aiding the global cranial implants market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the global cranial implants market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Zimmer Biomet Holdings, Inc.
- Medartis AG
- Medtronic Plc.
- Ortho Baltic, UAB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
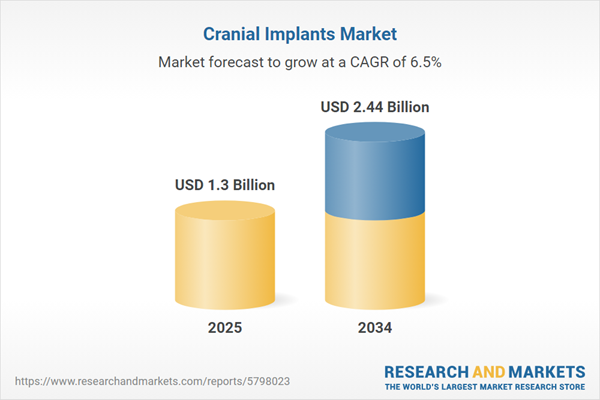
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 2.44 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |