Cardiovascular and Soft Tissue Repair Patches Market Overview
Cardiovascular and soft tissue repair patches are specialised medical devices designed to support tissue regeneration and healing in cardiac and soft tissue procedures. These patches, made from biological or synthetic materials, are used in surgeries to reinforce weakened or damaged tissues, such as in congenital heart defects, vascular repairs, and hernia treatments. They promote cell integration, reducing complications and improving patient outcomes. With advancements in biomaterials and regenerative medicine, these patches offer enhanced biocompatibility, durability, and adaptability, making them essential in cardiovascular and reconstructive surgeries worldwide.Cardiovascular and Soft Tissue Repair Patches Market Growth Drivers
Advanced Biomaterial Innovations to Drive Market Growth
The rising demand for biocompatible and multifunctional soft tissue repair patches is driven by increasing surgical procedures and advancements in biomaterial technology. The need for patches that reduce post-surgical adhesions, enhance healing, and lower inflammation is a key market driver. For instance, in May 2024, researchers introduced a Janus nanofibrous patch (J-NFP) with anti-adhesion, reactive oxygen species (ROS) scavenging, and pro-healing properties. Developed using sequential electrospinning and polymer grafting, the patch significantly reduces friction, protein adhesion, and inflammation while promoting tissue regeneration. This breakthrough is expected to expand the cardiovascular and soft tissue repair patches market, offering next-generation solutions for post-surgical healing and driving technological advancements in regenerative medicine over the forecast period.Cardiovascular and Soft Tissue Repair Patches Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Technological Advancements to Drive the Market Growth
The integration of advanced biomaterials and regenerative medicine is significantly enhancing cardiovascular and soft tissue repair patches. Innovations such as bioengineered patches with improved biocompatibility, durability, and regenerative properties are transforming surgical outcomes. The development of decellularised and tissue-engineered patches ensures better cell integration, reducing complications like fibrosis or immune rejection. Additionally, 3D bioprinting is revolutionising personalised medicine by enabling the production of customised patches tailored to individual patient needs. These advancements are expected to boost market demand, as they enhance surgical success rates and expand the applicability of these patches in various medical procedures.Increasing Prevalence of Cardiovascular Diseases to Boost Cardiovascular and Soft Tissue Repair Patches Market Demand
The rising incidence of cardiovascular diseases (CVDs), including congenital heart defects and vascular disorders, is a key driver of the market. Lifestyle-related conditions such as obesity, diabetes, and hypertension are contributing to the growing demand for advanced cardiac repair solutions. Furthermore, a surge in paediatric and geriatric patients requiring cardiovascular surgeries is increasing the need for effective tissue repair patches. As healthcare providers focus on early interventions and minimally invasive procedures, the market is expected to expand significantly, offering new opportunities for innovation in cardiovascular and reconstructive surgery.
Rising Adoption of Biological Patches to Accelerate Cardiovascular and Soft Tissue Repair Patches Market Value
The shift towards biological patches over synthetic alternatives is gaining momentum due to their superior biocompatibility and reduced risk of immune rejection. Biological patches derived from bovine, porcine, or human tissues provide enhanced healing capabilities, integrating seamlessly with the patient’s native tissue. Additionally, decellularization technology is improving patch longevity and safety, making them a preferred choice for cardiovascular and soft tissue repair. As regulatory approvals increase and research supports their long-term effectiveness, the demand for biological patches is expected to rise, driving market growth and expanding treatment options in cardiovascular and general surgery.
Growing Healthcare Investments and R&D Initiatives to Strengthen Cardiovascular and Soft Tissue Repair Patches Market Size
Increasing investments in healthcare infrastructure and R&D are accelerating the growth of the market. Governments and private organisations are funding studies to enhance biomaterials, improve surgical techniques, and develop next-generation repair patches. Additionally, collaborations between medical device companies and research institutions are leading to breakthrough innovations. As emerging economies invest in advanced medical technologies and expand healthcare access, the market is projected to witness substantial growth, ensuring better patient outcomes and greater adoption of soft tissue and cardiovascular repair solutions globally.
Cardiovascular and Soft Tissue Repair Patches Market Segmentation
Cardiovascular and Soft Tissue Repair Patches Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Material Type
- EPTFE
- Biomaterial
- Tissue Engineered Material
Market Breakup by Application
- Vascular Repair
- Pericardial Repair
- Dural Repair
- Soft Tissue Repair
Market Breakup by End User
- Hospitals
- Speciality Clinics
- Other End-Users
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Cardiovascular and Soft Tissue Repair Patches Market Share
Biomaterials to Lead the Segmentation by Material Type
Biomaterial-based patches are poised to dominate the market due to their superior biocompatibility, reduced risk of immune rejection, and enhanced regenerative properties. These patches integrate seamlessly with native tissues, promoting faster healing while minimising complications. As per the analysis by Expert Market Research, the global biomaterial market is likely to grow further at a CAGR of 14.10% during the forecast period of 2025-2034. Advancements in decellularized and bioengineered materials have further increased their adoption in cardiovascular and soft tissue procedures. The growing preference for biological over synthetic materials, coupled with increasing R&D investments in biomaterial innovation, is expected to drive market expansion, ensuring improved surgical outcomes and long-term patient benefits.Vascular Repair to Lead the Cardiovascular and Soft Tissue Repair Patches Market Segmentation by Application
Vascular repair is anticipated to hold the largest market share, driven by the rising prevalence of cardiovascular diseases, including aneurysms, arterial blockages, and congenital heart defects. The increasing number of vascular surgeries, particularly among the ageing population and patients with lifestyle-related disorders, is fuelling demand for effective repair solutions. Advanced vascular patches with improved flexibility, durability, and biocompatibility are enhancing surgical outcomes. Additionally, technological advancements such as 3D bioprinted vascular grafts and minimally invasive vascular repair techniques are further propelling market growth, making vascular repair the most dominant application segment in the forecast period.
Hospitals to Hold a Substantial Cardiovascular and Soft Tissue Repair Patches Market Share by End User
Hospitals are expected to dominate the market due to their extensive infrastructure, availability of advanced surgical equipment, and a high volume of complex cardiovascular and soft tissue repair procedures. The growing burden of cardiovascular diseases, rising patient admissions, and an increasing number of surgical interventions are boosting demand for high-quality repair patches in hospital settings. Additionally, government funding, technological advancements, and collaborations between hospitals and medical device manufacturers are driving innovations in surgical techniques. With a continuous focus on improving patient outcomes and expanding healthcare accessibility, hospitals will remain the primary end user, significantly influencing market growth.Cardiovascular and Soft Tissue Repair Patches Market Analysis by Region
North America is projected to hold the largest market share, driven by its advanced healthcare infrastructure, strong presence of key industry players, and high adoption of cutting-edge surgical technologies. The United States leads in cardiac and soft tissue procedures, supported by extensive R&D funding and favourable reimbursement policies. Europe follows closely, benefiting from increasing regulatory approvals and government initiatives promoting biomaterial-based patches. Asia Pacific is witnessing rapid growth due to rising medical tourism, improving healthcare accessibility, and expanding manufacturing capabilities.Leading Players in the Cardiovascular and Soft Tissue Repair Patches Market
The key features of the market report comprise patent analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:CryoLife, Inc
Founded in 1984 and headquartered in Kennesaw, Georgia, USA, CryoLife, Inc. is a leading medical device company specialising in cardiovascular and soft tissue repair solutions. The company focuses on the development and commercialisation of implantable human tissues, surgical adhesives, and prosthetic heart valves. CryoLife’s portfolio includes biological patches for vascular and cardiac repair, along with advanced haemostatic sealants for surgical applications. With a strong emphasis on innovation and research, CryoLife continues to enhance patient outcomes by providing high-quality, life-saving medical technologies to healthcare institutions worldwide.Edwards Lifesciences Corporation
Headquartered in Irvine, California, USA, Edwards Lifesciences Corporation was founded in 1958 and is a global leader in structural heart disease treatments and critical care monitoring. The company specialises in heart valve replacement therapies, including pericardial patches for cardiovascular repair. Edwards Lifesciences' extensive product portfolio includes transcatheter and surgical heart valves, tissue-engineered repair patches, and haemodynamic monitoring solutions. With continuous advancements in cardiac surgery, the company plays a key role in improving patient survival and quality of life, focusing on innovation, clinical leadership, and patient-centric healthcare solutions.Bard Peripheral Vascular Inc
Bard Peripheral Vascular Inc., a subsidiary of Becton, Dickinson and Company (BD), is headquartered in Tempe, Arizona, USA. The company has been a pioneer in vascular surgery solutions, offering an extensive range of products, including vascular grafts, surgical patches, and haemodialysis access solutions. With a strong focus on peripheral vascular disease treatment, Bard Peripheral Vascular provides high-quality biomaterial-based patches for arterial and venous repair. Through continuous innovation and collaboration with healthcare professionals, the company aims to improve patient outcomes in cardiovascular and soft tissue repair procedures.Baxter International Inc
Established in 1931 and headquartered in Deerfield, Illinois, USA, Baxter International Inc. is a global leader in healthcare, offering innovative medical devices, pharmaceuticals, and biotechnology solutions. The company’s portfolio includes advanced haemostatic agents, biological patches for cardiovascular and soft tissue repair, and surgical sealants. Baxter focuses on improving surgical efficiency and patient recovery through its cutting-edge biomaterials and regenerative medicine products. With a strong presence across hospitals and healthcare institutions worldwide, Baxter continues to drive advancements in surgical care through research-driven solutions and medical technology innovation.Anteris Technologies Limited
Anteris Technologies Limited, headquartered in Brisbane, Australia, was founded in 1999 and specialises in regenerative medicine and structural heart solutions. The company focuses on the development of next-generation biomaterials and tissue-engineered patches for cardiovascular repair. Its flagship offerings include advanced pericardial patches and implantable cardiac devices designed to enhance heart valve performance and durability. With a strong commitment to innovation and patient-centred research, Anteris Technologies is at the forefront of developing novel solutions for heart valve disease, significantly contributing to the advancement of cardiovascular care worldwide.Other key players in the market include Shockwave Medical, Inc., Southern Lights Biomaterials (Collagen Solutions), Medtronic Plc, GETINGE AB, W. L. Gore & Associates, Inc., LeMaitre Vascular Inc., and B. Braun Melsungen AG.
Key Questions Answered in the Cardiovascular and soft tissue repair patches Market
- What was the global cardiovascular and soft tissue repair patches market value in 2024?
- What is the cardiovascular and soft tissue repair patches market forecast outlook for 2025-2034?
- What is market segmentation based on material type?
- What is market breakup based on application?
- What is market segmentation based on end users?
- What are the major factors aiding the cardiovascular and soft tissue repair patches market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major cardiovascular and soft tissue repair patches market trends?
- Which material type will lead the market segment?
- Which application will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the cardiovascular and soft tissue repair patches market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- CryoLife, Inc.
- Edwards Lifesciences Corporation
- Bard Peripheral Vascular Inc.
- Baxter International Inc.
- Anteris Technologies Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
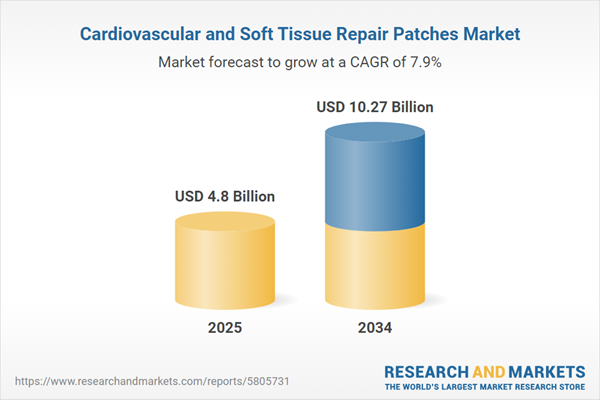
| Estimated Market Value ( USD | $ 4.8 Billion |
| Forecasted Market Value ( USD | $ 10.27 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |