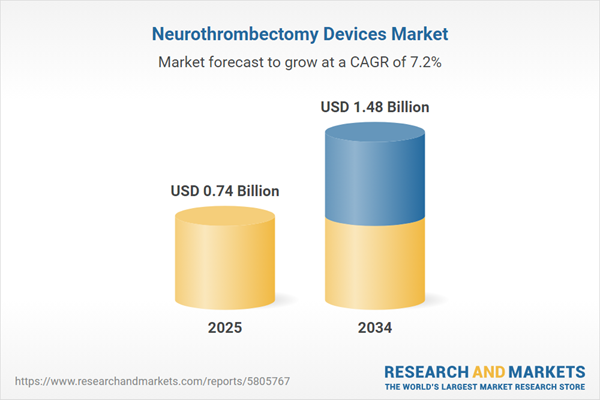
Neurothrombectomy Devices Market Overview
Neurothrombectomy devices are medical tools used to remove blood clots from cerebral arteries in patients experiencing acute ischemic stroke. These devices restore blood flow by mechanically retrieving or aspirating clots, reducing stroke-related disability. Key types include stent retrievers and aspiration catheters, often used alongside thrombolytic therapy. Advancements in catheter design, imaging technology, and minimally invasive techniques have improved success rates and patient outcomes. With increasing stroke cases globally, neurothrombectomy devices play a crucial role in emergency stroke care, offering faster clot removal and improved survival rates compared to traditional treatment methods.Neurothrombectomy Devices Market Growth Drivers
Rising Demand for Advanced Catheter Systems to Drive Market Growth
The market is expanding due to the increasing incidence of ischemic strokes and advancements in catheter-based interventions. Minimally invasive procedures are becoming the standard of care, boosting demand for high-precision neurovascular tools. For instance, in July 2024, Route 92 Medical, Inc. launched the FreeClimb® 88 catheter system, cleared by the FDA (510(k)), featuring improved flexibility and navigation through complex neurovasculature. This development is set to drive market growth by enhancing procedural success rates, reducing complications, and providing physicians with greater accessibility to challenging anatomical locations. As healthcare providers increasingly adopt innovative neurovascular technologies, demand for next-generation catheter systems will continue to fuel the market’s expansion during the forecast period.Technological Advancements in Balloon Guide Catheters to Enhance Neurothrombectomy Devices Market Demand
The market is driven by the rising adoption of mechanical thrombectomy (MT) and continuous advancements in endovascular technologies. For instance, in September 2024, CERENOVUS, Inc. (Johnson & Johnson MedTech) introduced the EMBOGUARD Balloon Guide Catheter, designed to optimize clot removal during MT procedures. This device improves first-pass recanalization rates, reduces procedural time, and minimizes the risk of clot fragmentation, which can cause secondary ischemic events. The launch of next-generation balloon guide catheters is expected to accelerate market development by enhancing procedural efficiency, improving patient outcomes, and strengthening the adoption of MT in stroke treatment protocols. As demand for effective stroke management solutions rises, the market is poised for significant advancements and innovation.
Neurothrombectomy Devices Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Innovative Technologies to Enhance Market Growth
The market is experiencing substantial growth due to rapid technological advancements in clot retrieval mechanisms, real-time imaging, and AI-powered stroke detection. Innovations like stent retrievers with enhanced flexibility and aspiration-based thrombectomy systems are improving procedural success rates. The increasing integration of robotics and AI-driven diagnostics is also expanding treatment precision and accessibility. These advancements are driving higher adoption rates among neurosurgeons and interventional radiologists, leading to improved patient outcomes. As research continues to refine neurothrombectomy procedures, the market is expected to grow significantly, catering to the rising global burden of ischemic strokes.Strategic Collaborations to Impact Neurothrombectomy Devices Market Size Positively
The market is developing robustly due to strategic partnerships between medical device manufacturers, healthcare institutions, and research organisations. Companies are collaborating to enhance product innovation, regulatory approvals, and clinical trial efficiency, ensuring safer and more effective devices reach the market. Public-private partnerships are also fostering funding for R&D and AI-based stroke management solutions. Additionally, mergers and acquisitions among key players are strengthening market presence and distribution networks. Such collaborations are accelerating technological advancements and market expansion, enabling broader accessibility to advanced neurothrombectomy solutions worldwide.Rising Stroke Incidence to Boost Neurothrombectomy Devices Market Demand
The increasing global prevalence of ischemic strokes, primarily due to ageing populations and lifestyle-related risk factors, is significantly boosting the market value of neurothrombectomy devices. Currently, 1 in 4 adults over 25 will experience a stroke in their lifetime, with over 12 million first-time strokes occurring annually and 6.5 million resulting in death. While stroke incidence rises with age, over 60% affect individuals under 70, and 16% occur in those under 50. As strokes remain a leading cause of disability and mortality, healthcare providers are adopting advanced thrombectomy procedures, alongside growing reimbursement policies and government investments, further driving market expansion.Expanding Product Portfolios to Drive Neurothrombectomy Devices Market Value
A key trend in the neurothrombectomy devices market is the expansion of product portfolios by major industry players to address the growing demand for advanced neurovascular interventions. Companies are continuously developing innovative access and thrombectomy solutions to enhance procedural success rates and improve stroke patient outcomes. For instance, in June 2024, Penumbra received CE Mark approval and launched BMX™81 and BMX™96 in Europe, designed for neurovascular management of ischemic and hemorrhagic strokes. This follows the company’s May 2024 launch of RED™ reperfusion catheters. These developments strengthen market competition, expand treatment options for physicians, and accelerate the adoption of minimally invasive neurovascular technologies, driving global market growth in the forecast period.Neurothrombectomy Devices Market Segmentation
Neurothrombectomy Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Stent Relievers
- Aspiration Systems
- Clot Extractors
- Combination Systems
Market Breakup by Application
- Acute Ischemic Stroke
- Other Neurovascular Conditions
Market Breakup by End User
- Hospitals
- Outpatient Clinics
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Neurothrombectomy Devices Market Share
Stent Retrievers to Dominate the Segment by Product
Stent retrievers are poised to hold the largest market share due to their high efficacy in restoring blood flow in acute ischemic stroke patients. Their minimally invasive nature, rapid clot removal capabilities, and proven clinical outcomes drive adoption. Technological advancements in self-expanding designs enhance their efficiency, reducing procedure time and complications. Increasing stroke prevalence, rising emergency procedures, and growing awareness of mechanical thrombectomy further fuel demand. With healthcare facilities prioritising innovative stroke treatment solutions, stent retrievers will continue to dominate, supported by ongoing clinical trials, favourable regulatory approvals, and expanding indications for neurovascular interventions.Acute Ischemic Stroke to Leading the Neurothrombectomy Devices Market Segmentation by Application
Acute ischemic stroke (AIS) is set to hold the largest market share, driven by its high global incidence and urgent need for rapid intervention. Neurothrombectomy devices play a critical role in reducing disability and mortality when used within the golden treatment window. As per the analysis by Expert Market Research, the ischemic stroke market is expected to grow at a CAGR of 5.2% during the forecast period of 2025-2034 . Increasing adoption of mechanical thrombectomy, advancements in stroke imaging, and rising healthcare investments support growth. Additionally, government initiatives promoting stroke awareness and treatment accessibility enhance market penetration. With a growing ageing population, rising risk factors like hypertension and diabetes, and expanding emergency care networks, the AIS segment is expected to drive substantial market expansion.Hospitals to Lead the Neurothrombectomy Devices Market by End User
Hospitals are expected to dominate the market due to their advanced infrastructure, availability of neurointerventional specialists, and increasing stroke admissions. As primary centres for acute stroke treatment, hospitals benefit from favourable reimbursement policies, ongoing clinical trials, and access to cutting-edge thrombectomy technologies. The shift towards comprehensive stroke centres and the expansion of neurovascular units further strengthens hospital dominance. Increasing public and private healthcare investments, along with growing adoption of AI-assisted imaging and robotic-assisted thrombectomy, enhance treatment efficiency. With continuous advancements in stroke care protocols, hospitals will remain the leading segment, driving overall market growth and innovation.Neurothrombectomy Devices Market Analysis by Region
North America is set to dominate the devices market, driven by high healthcare expenditure, advanced medical infrastructure, and increasing stroke prevalence. The region benefits from technological advancements, favourable reimbursement policies, and strong regulatory support for neurovascular devices. Rising awareness of early stroke intervention and an ageing population further fuel market growth. Additionally, key players are investing in R&D and expanding product portfolios, strengthening market penetration. With a growing focus on minimally invasive stroke treatment and increasing adoption of mechanical thrombectomy devices, North America remains the largest and fastest-growing regional market for neurothrombectomy devices.Leading Players in the Neurothrombectomy Devices Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Medtronic plc
Headquartered in Dublin, Ireland, Medtronic plc was founded in 1949 and is a global leader in medical technology. The company’s neurothrombectomy portfolio includes Solitaire™ X Revascularisation Device, a widely used stent retriever for acute ischemic stroke treatment. Medtronic focuses on minimally invasive therapies, offering advanced stroke management solutions that enhance patient outcomes. With a strong presence in the North American market, Medtronic continues to innovate in neuroscience technologies, supporting early intervention and improved recanalisation rates for patients suffering from large vessel occlusions (LVOs).Stryker Corporation
Established in 1941 and headquartered in Kalamazoo, Michigan, USA, Stryker Corporation is a leading provider of medical devices and stroke care solutions. Its neurothrombectomy product line includes the Trevo® NXT ProVue Retriever, designed for rapid clot removal in ischemic stroke patients. The company specialises in neurovascular innovations, offering stent retrievers, aspiration catheters, and integrated stroke care technologies. In September 2024, Stryker acquired NICO Corporation, enhancing its neurotechnology portfolio with minimally invasive surgical solutions for intracerebral hemorrhage (ICH) and tumor procedures, strengthening stroke and neurovascular care. Stryker’s commitment to research and development drives continuous advancements in neurointervention, supporting improved revascularisation, procedural efficiency, and patient outcomes in the acute ischemic stroke treatment market.Penumbra, Inc
Founded in 2004 and headquartered in Alameda, California, USA, Penumbra, Inc. is a key player in neurovascular intervention technologies. The company’s REAL™ Revascularisation System and Penumbra JET® aspiration catheters are widely used for mechanical thrombectomy in acute ischemic stroke. In June 2024, Penumbra launched BMX™81 and BMX™96 in Europe for neurovascular stroke management, expanding its advanced neuro access portfolio. Penumbra is known for its aspiration-based thrombectomy solutions, which aim to enhance clot removal efficiency while minimising complications. With a strong focus on continuous innovation and physician collaboration, Penumbra continues to advance minimally invasive stroke treatments, supporting improved clinical outcomes and patient recovery in the market.Cerenovus (a Johnson & Johnson company)
A subsidiary of Johnson & Johnson, Cerenovus is headquartered in Irvine, California, USA, and focuses on neurovascular technologies. The company offers advanced neurothrombectomy solutions, including the EMBOTRAP® III Revascularisation Device and CEREBASE™ DA Guide Sheath, designed for efficient clot retrieval in ischemic stroke. With a commitment to stroke care innovation, Cerenovus integrates research-driven design, biomaterials expertise, and clinical insights to improve patient outcomes. Backed by Johnson & Johnson’s extensive healthcare network, Cerenovus continues to expand its neurovascular portfolio, providing cutting-edge thrombectomy devices for stroke treatment worldwide.Other key players in the market include Boston Scientific Corporation, AngioDynamics, Inc., Terumo Corporation, Acandis GmbH, Phenox GmbH, and Vesalio, LLC.
Key Questions Answered in the Neurothrombectomy Devices Market
- What was the global neurothrombectomy devices market value in 2024?
- What is the global neurothrombectomy devices market forecast outlook for 2025-2034?
- What is market segmentation based on product?
- What is market segmentation based on application?
- What is market segmentation based on end users?
- What are the major factors aiding the global neurothrombectomy devices market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major global neurothrombectomy devices market trends?
- Which product will lead the market segment?
- Which application will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the global neurothrombectomy devices market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Medtronic plc
- Stryker Corporation
- Penumbra, Inc.
- Cerenovus (a Johnson & Johnson company)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 0.74 Billion |
| Forecasted Market Value ( USD | $ 1.48 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |