Asia Pacific is the promising region for cervical cancer diagnostic because rise in the prevalence of obesity among women, an increase in instances of hazardous sexual behavior, particularly among adolescents, and an increase in the number of diverse strategies and trends adopted by market participants. Hence, APAC generated $1,929.4 million revenue in the market in 2022. Numerous public-private partnerships, rising R&D efforts, and strategic initiatives by market participants are creating opportunities for the market in the region.
The major strategies followed by the market participants are Partnerships as the key developmental strategy to keep pace with the changing demands of end users. For instance,In July 2021, QIAGEN partnered with Sysmex Corporation for developing cancer companion diagnostics by utilizing Qiagen's expertise and Sysmex’s Plasma-Safe-SeqS technology. The partnership provides the company with NGS capabilities and would allow the company to serve its partners in a better way. Additionally, In August 2022, Becton, Dickinson, and Company announced a partnership with LabCorp for developing and selling flow cytometry-based companion diagnostics (CDx). The partnership allows BD to serve its customers in a better way by providing solutions for cancer diagnostics.
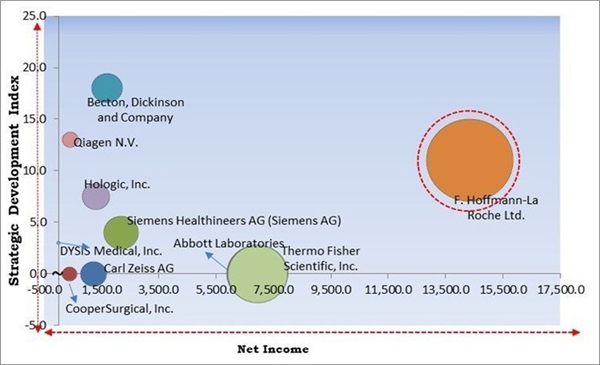
The Cardinal Matrix - Market Competition Analysis
Based on the Analysis presented in the The Cardinal Matrix; F. Hoffmann-La Roche Ltd. is the forerunner in the Market. In May 2023, Roche Diagnostics signed an agreement with Cancer Awareness Prevention and Early Detection Trust (CAPED) for improving the situation of cervical cancer in India. The partnership is part of Roche's efforts to enhance cancer care around the world. Companies such as Abbott Laboratories, Thermo Fisher Scientific, Inc., Becton, Dickinson and Company are some of the key innovators in Market.
Market Growth Factors
Increasing initiatives for the treatment and diagnosis of cancer
Healthcare expenditures have increased globally as disposable income has risen in various countries. In addition, government bodies and healthcare organizations are accelerating healthcare expenditures to meet population requirements. As cervical cancer has become increasingly prevalent in recent years, the increase in healthcare expenditures enables healthcare institutions to enhance their diagnostic and therapeutic facilities for the disease. In addition, key market participants' strategic initiatives will provide structural integrity and future growth opportunities for the market.Rising cases of cervical cancer
If detected early and managed effectively, cervical cancer is one of the most successfully treatable forms once diagnosed. Late-stage cancers can also be controlled with the correct treatment and palliative care. Cervical cancer can be eliminated within a single generation if enough measures are taken in prevention, screening, and treatment. Several advanced laboratory tests, instruments, and procedures that evaluate abnormal cells and strains of the human papillomavirus (HPV) are used to diagnose cervical cancer. Consequently, the growing incidence of cervical cancer and its treatability following early diagnosis will increase the demand.Market Restraining Factors
A lack of qualified medical personnel
By 2035, there will likely be a shortage of 12.9 million healthcare workers, an increase from the 7.2 million that exist today. According to recent WHO research, the lack of healthcare workers might have a catastrophic impact on the health of billions of people worldwide if prompt actions are not taken. One reason contributing to this situation is the aging of the workforce, which causes retirements or leaves for higher-paying employment without comparable replacements. Additionally, the sector lacks fresh hires and offers inadequate training. The identification and treatment of illnesses like cervical cancer would be significantly impacted by a global scarcity of healthcare experts, which would impede the market growth.Type Outlook
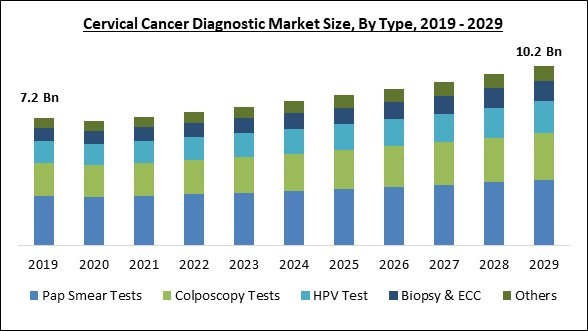
Based on type, the market is segmented into pap smear tests, HPV test, biopsy & ECC, colposcopy tests and others. The pap smear tests segment dominated the market with maximum revenue share in 2022. This is because more people are using Pap smear tests to diagnose cervical cancer. The Pap test aids in identifying abnormal cervix cells that may later progress to cancer. Due to this test's great efficiency, it makes up the majority of the shares. Additionally, this segment is growing as early diagnosis is becoming increasingly popular.Age Group Outlook
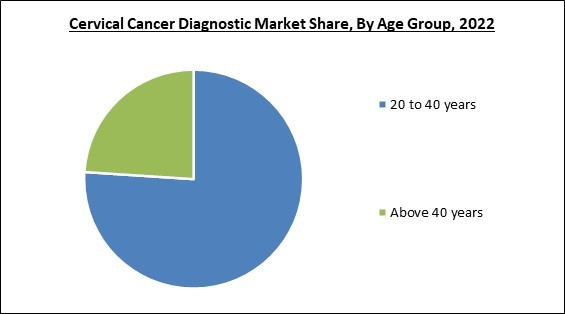
On the basis of age group, the market is divided into 20 to 40 years and above 40 years. The above 40 years segment procured a substantial revenue share in the market in 2022. This is owing to the increased government support for cervical cancer diagnosis. Many elderly women are unaware that the risk of developing cervical cancer persists with age, increasing the demand and importance of diagnosis. In addition, approximately one-fifth of cervical cancer cases are diagnosed in women aged 65 and older, propelling the segment's expansion.Regional Outlook
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region witnessed the largest revenue share in the market in 2022. This is due to the increasing number of patients utilizing cervical cancer diagnosis services in hospitals, an increase in the number of market participants, joined with the rising availability of products in the region. In addition, the large market share is attributable to the high level of disease prevention awareness among women in the region, as well as the numerous initiatives introduced to prevent cervical cancer, which has expanded insurance coverage for cervical screening tests, particularly for low-income women, which is aiding the market growth in the region.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Becton, Dickinson and Company, Carl Zeiss AG, CooperSurgical, Inc. (The Cooper Companies, Inc.), DYSIS Medical, Inc., F. Hoffmann-La Roche Ltd., Hologic, Inc., Qiagen N.V., Siemens Healthineers AG (Siemens AG) and Thermo Fisher Scientific, Inc.
Strategies Deployed in the Market
Partnerships, Collaborations and Agreements:
- May-2023: Roche Diagnostics signed an agreement with Cancer Awareness Prevention and Early Detection Trust (CAPED), a non-profit organization aiming at spreading Cancer Awareness. The partnership aims at improving the situation of cervical cancer in India. The partnership is part of Roche's efforts to enhance cancer care around the world.
- Aug-2022: Becton, Dickinson, and Company announced a partnership with LabCorp, a life science company. The partnership aims at developing and selling flow cytometry-based companion diagnostics (CDx). The partnership allows BD to serve its customers in a better way by providing solutions for cancer diagnostics.
- Jul-2021: QIAGEN partnered with Sysmex Corporation, a healthcare company based in Japan. The partnership aims at developing cancer companion diagnostics by utilizing Qiagen's expertise and Sysmex’s Plasma-Safe-SeqS technology. The partnership provides the company with NGS capabilities and would allow the company to serve its partners in a better way.
- Feb-2021: Hologic, Inc. partnered with Google Cloud, a range of cloud-based services offered by Google. The partnership aims at enhancing cancer screening and removal of cervical cancer around the world by integrating google cloud's machine learning (MI) technology with Holigic's Genius™ Digital Diagnostics System. The partnership enhances Hologic's position as a premier provider of cancer screening solutions.
Product Launches and Product Expansions:
- May-2023: Becton, Dickinson, and Company unveiled BD FACSDiscover S8 Cell Sorter. BD FACSDiscover S8 Cell Sorter is used for detailed profiling of cells. The BD FACSDiscover S8 Cell Sorter features BD CellView Image Technology and BD SpectralFX Technology. The BD CellView Image Technology is used for cell characteristics sorting by providing the researchers with high-quality images of cells. The With BD SpectralFX Technology is used for full range cell sorting using AI-based algorithms and optical architecture.
- Apr-2023: QIAGEN unveiled QIAseq Targeted cfDNA Ultra Panels. The QIAseq Targeted cfDNA Ultra Panels were designed for use by researchers for converting cfDNA liquid biopsy samples into libraries for further use in next-generation sequencing (NGS).
- Jun-2022: Roche unveiled cobas HPV. The cobas HPV is a human papillomavirus (HPV) self-sampling solution that is designed for use by female patients to collect their samples.
- Jul-2021: DYSIS Medical Ltd. announced the launch of DYSIS View. The DYSIS View is used for finding out cervical lesions. The DYSIS view features cervical mapping technology, DYSIS SMARTtrack, and a camera used for high-quality images and exam videos.
- Aug-2021: Becton, Dickinson, and Company unveiled BD COR System, a self-controlled diagnostic system used for automating molecular laboratory workflow. The system features scalability, modularity, and onboard capacity for reagents. Furthermore, the system features a PX instrument, used for preparing samples, and GX instrument used for performing analysis.
- Apr-2021: Hologic, Inc. unveiled Genius Digital Diagnostics System. Genius Digital Diagnostics System is used for detecting cervical cancer. The Genius Digital Diagnostics System features ThinPrep Pap test image to provide a single view image of relevant cells and GeniusTM Cervical AI that is used in sorting cells to AI-generated gallery.
Trials and approvals:
- Feb-2023: Becton, Dickinson, and Company have received approval from U.S. Food and Drug Administration (FDA) for its BD Onclarity™ HPV Assay. The BD Onclarity™ HPV Assay is used to detect human papillomavirus (HPV).
- May-2021: Hologic received premarket approval for its ThinPrep GenesisTM processor. ThinPrep GenesisTM processor is used for specimen transfer and cytology processing applications. The features of the product include barcode scanning, sample aliquoting, slide labelling, and vial uncapping/capping.
Acquisition and Mergers:
- Jul-2022: Becton, Dickinson, and Company acquired Parata Systems, a pharmacy automation solutions provider. The acquisition aids the company in its growth strategy and allows the company to expand into the high-growth pharmacy automation sector.
- Feb-2022: Becton, Dickinson, and Company acquired Cytognos, a flow cytometry solutions provider. The acquisition provides Becton, Dickinson, and Company with Euroflow Consortium which further allows the company to expand their capabilities into Post treatment monitoring.
- May-2021: Roche announced the acquisition of GenMark Diagnostics, a molecular diagnostics company. The acquisition expands Roche's molecular diagnostics portfolio and enhances its capabilities in syndromic testing.
- Apr-2021: Siemens Healthineers AG acquired Varian Medical Systems, Inc., a radiation oncology treatments provider. This acquisition places Siemens Healthineers AG as a major player in the MedTech sector.
Geographical Expansions:
- Mar-2022: Hologic opened an innovation centre in France. The new centre would serve as a research and development and training hub for the company. The new centre aids the company in providing new technologies to the European market.
Scope of the Study
By Type
- Pap Smear Tests
- Colposcopy Tests
- HPV Test
- Biopsy & ECC
- Others
By Age Group
- 20 to 40 years
- Above 40 years
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Abbott Laboratories
- Becton, Dickinson and Company
- Carl Zeiss AG
- CooperSurgical, Inc. (The Cooper Companies, Inc.)
- DYSIS Medical, Inc.
- F.Hoffmann-La Roche Ltd.
- Hologic, Inc.
- Qiagen N.V.
- Siemens Healthineers AG (Siemens AG)
- Thermo Fisher Scientific, Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Becton, Dickinson and Company
- Carl Zeiss AG
- CooperSurgical, Inc. (The Cooper Companies, Inc.)
- DYSIS Medical, Inc.
- F. Hoffmann-La Roche Ltd.
- Hologic, Inc.
- Qiagen N.V.
- Siemens Healthineers AG (Siemens AG)
- Thermo Fisher Scientific, Inc.