H. pylori is a bacterium causing stomach infection, peptic ulcer, and gastritis. The prolonged bacterial infection may lead to stomach cancer in some cases. The H. pylori infection is more common in the US Hispanic and black populations, which can be credited to socioeconomic factors such as low income and literacy, household crowding, and large-scale immigration. Thus, increase in prevalence of H. pylori infection fuel the US Helicobacter pylori non-invasive testing market.
The H. pylori infection usually occurs in children with long-standing clinical symptoms such as gastritis, peptic ulcer disease, and stomach cancer. According to an article published in the Nature Journal, H. pylori is present in ~36% of the US population, posing a significant risk for gastric cancer and peptic ulcer disease. The World Health Organization (WHO) has recognized H. pylori as a carcinogen that leads to gastric cancer and in the US, gastric cancer is the third most common gastrointestinal cancer. According to the American Cancer Society, nearly 26,500 new cases of stomach cancer and ~11,130 deaths will occur due to this cancer type in 2023. Thus, growing cancer cases increases the US Helicobacter pylori non-invasive testing market growth.
Further, the growing geriatric population pool susceptible to gastric diseases will substantially increase the risk of H. pylori infection. Aging leads to thinning of the stomach lining, thus allowing the bacteria to pass through the protective lining, thereby increasing the disease prevalence among older people. Given the constant increase in the geriatric population base, the Washington Post has estimated that there will be nearly 80.8 million senior citizens in the US by 2040. Thus, growing geriatric population boosts the growth of US Helicobacter pylori non-invasive testing market.
According to a report from the National Institutes of Health, there was a 5% prevalence of H. pylori in children under the age of 10 in the US in 2020. In addition, the US Department of Health and Human Services reports that 30-40% of Americans are infected with H. pylori infection. Further, socioeconomic factors, such as low income, lack of education, household crowding, and immigration contribute to H. pylori infection among Hispanic and Black populations in the US. These statistics determine an increase in the need for non-invasive diagnostics for H. pylori in the US, thus, boosting the growth of the US Helicobacter pylori non-invasive testing market.
Test Type Insights
Based on test type, the US Helicobacter pylori non-invasive testing market is categorized into urea breath, stool antigen, and serology. In 2022, the urea breath segment held the largest share of the US Helicobacter pylori non-invasive testing market. Moreover, the same segment is expected to grow at the fastest rate during the coming years. A urea breath test (UBT) is one of the essential noninvasive methods for detecting H. pylori infection. The test involves detecting H. pylori based on its ability to produce urease enzyme, which results in the hydrolysis of orally administered urea into carbon dioxide and ammonia. These gases diffuse into the blood, and the lungs then excrete them. UBTs are reliable, low-burden tests validated in adults and children. Thus, these contribute to the growth of US Helicobacter pylori non-invasive testing market size.According to Cancer Research UK, chronic bacterial infection causes long-lasting stomach inflammation, also known as severe chronic atrophic gastritis or SCAG; prolonged stomach ulcers can even lead to cancer. According to the World Health Organization (WHO), cancer is a significant cause of death across the world; approximately 1.9 million new cancer cases were recorded in the world in 2021. Colorectal cancer is a common type, with 146,589 new cases recorded in 2022. This type of cancer is more prevalent in middle-aged and geriatric populations. It rarely occurs among people aged less than 40 with genetic predisposition or predisposing conditions. The early detection of H. pylori via appropriate tests is necessary to prevent colorectal cancer. Early diagnosis is pivotal to reducing cancer-related mortality rates. Thus, the rising cases of bacterial infections are necessitating urea breath tests which contributes to the growth of US Helicobacter Pylori non-invasive testing market.
In February 2020, Gulf Coast Scientific received approval from the US Food and Drug Administration (FDA) for the Pylo Plus UBT System, a 13C UBT for the detection of H. pylori infection. In addition, key players in the US Helicobacter pylori non-invasive testing market form alliances to introduce new products and expand their presence. For instance, in 2021, Meridian Bio signed an agreement to acquire BreahTrek from Otsuka Pharmaceutical for US$ 20 million. Such collaborations led to the new product launches in the UBT segment and contribute the overall growth of US Helicobacter pylori non-invasive testing market during the forecast period.
Table of Contents
Companies Mentioned
- Bioneer Corp
- Meridian Bioscience Inc
- Abbott Laboratories
- Thermo Fisher Scientific Inc
- Bio-Rad Laboratories Inc
- Coris Bioconcept SPRL
- DiaSorin SpA
- Sekisui Diagnostics LLC
- CerTest Biotec SL
- QuidelOrtho Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 112 |
| Published | June 2023 |
| Forecast Period | 2023 - 2028 |
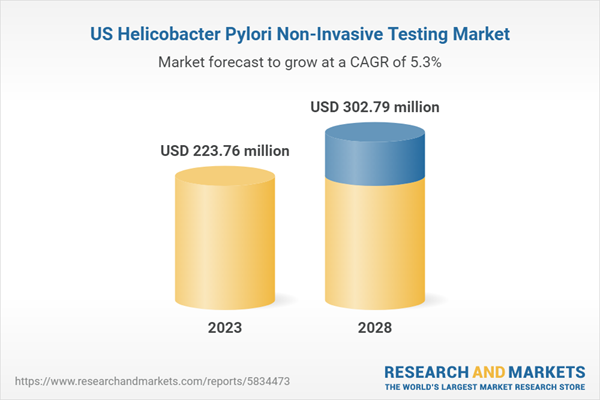
| Estimated Market Value ( USD | $ 223.76 Million |
| Forecasted Market Value ( USD | $ 302.79 Million |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |