Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these drivers, market progress faces significant hurdles due to the high costs involved in purchasing and maintaining medical-grade systems. This financial burden restricts the deployment of essential equipment in smaller clinics and lower-income areas, thereby limiting broader market penetration. Moreover, the need for specialized training to correctly analyze monitoring data creates operational bottlenecks. A global scarcity of skilled healthcare practitioners further complicates matters, preventing the widespread application of these critical safety protocols in standard medical practice.
Market Drivers
The market is being significantly propelled by breakthroughs in non-invasive and portable monitoring technologies, which allow for continuous fetal observation beyond the confines of traditional hospitals. These wireless devices monitor vital signs without keeping patients tethered to bedside machines, enhancing remote management capabilities and improving adherence to care plans.Such technological evolution permits the timely identification of complications in high-risk pregnancies without necessitating extended hospital stays, effectively balancing clinical safety with patient freedom. Illustrating this trend, GE HealthCare received FDA clearance in February 2024 for its Novii+ solution, expanding wireless monitoring options for a wider demographic. This rapid innovation is supported by strong financial backing; Silicon Valley Bank reported that venture capital investment in women’s health hit $2.6 billion in 2024, providing crucial capital for developing these advanced diagnostic tools.
Furthermore, government support for maternal and infant health serves as a vital engine for growth by directing funds toward modernizing obstetric infrastructure. Public health bodies are prioritizing spending to lower mortality rates and close healthcare gaps, facilitating the purchase of medical-grade equipment in rural and underserved communities.
These initiatives often subsidize the expense of sophisticated devices while enforcing strict safety standards to guarantee fair access to perinatal services. For instance, in October 2024, the Health Resources and Services Administration granted approximately $19 million to state-level projects designed to improve maternal health results and service availability. Such targeted public funding reduces economic obstacles for medical centers, encouraging the widespread adoption of necessary fetal assessment technologies.
Market Challenges
A major obstacle hindering the Global Fetal Monitoring Market is the necessity for specialized training alongside a worldwide lack of skilled healthcare workers. Fetal monitoring devices produce intricate physiological data, such as uterine contractions and fetal heart rate rhythms, which require high-level clinical knowledge for proper analysis. Failing to accurately differentiate between harmless variations and actual fetal distress can result in serious health consequences and substantial legal risks for healthcare providers. This reliance on expert interpretation generates a bottleneck, as the rollout of these diagnostic tools is limited by the availability of personnel capable of operating them and interpreting the results.Consequently, the shortage of qualified midwives and obstetricians directly limits the ability to scale monitoring services, especially in areas with severe workforce shortages. Many medical institutions are compelled to restrict the implementation of advanced monitoring systems because they lack adequate staff to uphold rigorous surveillance protocols. According to the American College of Obstetricians and Gynecologists, the supply of obstetrician-gynecologists in the United States satisfied only 93.4% of the demand in 2025. This expanding disparity between clinical needs and workforce availability impedes the universal application of these vital safety measures, effectively stalling the market's growth.
Market Trends
The incorporation of Artificial Intelligence (AI) into predictive fetal analytics is transforming the market, shifting focus from basic data recording to real-time risk assessment. Modern algorithms are capable of examining complex heart rate patterns to detect distress signals sooner, thereby aiding clinical decisions regarding preterm and high-risk pregnancies. This evolution is highlighted by regulatory achievements that confirm AI's utility in broadening surveillance to include more vulnerable stages of gestation. For example, PeriGen obtained FDA clearance in February 2025 for its Patterns 3.0 software, extending its AI-driven pattern recognition to fetuses as young as 32 weeks. These innovations enable medical teams to handle critical cases with higher accuracy, diminishing the dependence on subjective analysis that frequently causes delays in acute care environments.In parallel, the rise of smartphone-integrated smart monitoring tools is decentralizing prenatal services, empowering expectant mothers to conduct clinical-quality checks from home. These portable devices connect directly with mobile apps to send images and data to healthcare providers for remote evaluation, significantly lowering the frequency of required clinic visits. This shift toward consumer-oriented, connected healthcare was reinforced when Pulsenmore received FDA De Novo marketing authorization in November 2025 for its home-based prenatal ultrasound platform, which uses a smartphone dock to facilitate patient-guided scanning. This functionality effectively tackles the issue of care deserts by bridging the geographical gap between patients and obstetricians while ensuring a consistent flow of diagnostic-grade information.
Key Players Profiled in the Fetal Monitoring Market
- Medtronic PLC
- Cardinal Health Inc.
- Koninklijke Philips N.V.
- Siemens Healthineers AG
- GE Healthcare Technologies Inc.
- FUJIFILM SonoSite, Inc.
- Natus Medical Incorporated
- Huntleigh Healthcare Limited
- CooperCompanies Inc.
- CONTEC Medical Systems Co., Ltd.
Report Scope
In this report, the Global Fetal Monitoring Market has been segmented into the following categories:Fetal Monitoring Market, by Product:
- Ultrasound
- Device
- Fetal Monitors
- Uterine Contraction Monitors
- Fetal Electrodes
- Fetal Doppler Device
- Telemetry Device
- Accessories and Consumables
- Others
Fetal Monitoring Market, by Portability:
- Portable System and Non-Portable System
Fetal Monitoring Market, by Method:
- Invasive and Non-invasive
Fetal Monitoring Market, by Application:
- Antepartum
- Intrapartum
Fetal Monitoring Market, by End User:
- Hospitals
- Obstetrics & Gynecology clinic
- Home Care Setting
Fetal Monitoring Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Fetal Monitoring Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Fetal Monitoring market report include:- Medtronic PLC
- Cardinal Health Inc.
- Koninklijke Philips N.V.
- Siemens Healthineers AG
- GE Healthcare Technologies Inc.
- FUJIFILM SonoSite, Inc
- Natus Medical Incorporated
- Huntleigh Healthcare Limited
- CooperCompanies Inc.
- CONTEC Medical Systems Co., Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
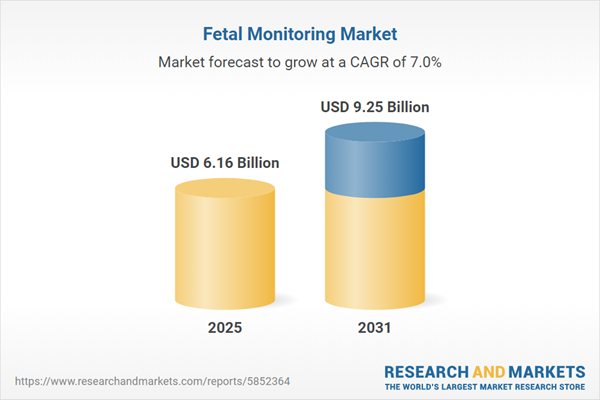
| Estimated Market Value ( USD | $ 6.16 Billion |
| Forecasted Market Value ( USD | $ 9.25 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |