Rapid Test Kit: Introduction
Rapid test kits, also known as rapid diagnostic tests (RDTs) or point-of-care tests, are medical devices used for the rapid detection and diagnosis of various diseases or conditions. These tests provide quick and convenient results, usually within minutes, enabling healthcare professionals to make immediate treatment decisions. Rapid test kits have gained significant popularity due to their simplicity, portability, and ability to deliver rapid results without the need for laboratory equipment or specialized training. Rapid test kits are designed to detect specific markers or antigens in biological samples, such as blood, urine, or saliva. They are commonly used for infectious diseases, including viral infections (such as HIV, influenza, and hepatitis), bacterial infections (such as streptococcus), and sexually transmitted infections. They can also be used for the detection of certain chronic conditions, such as diabetes or cardiac markers.Key Trends in the Global Rapid Test Kit Market
There are several key trends in the market and some of them are:- Increasing demand for point-of-care testing: Rapid test kits offer the advantage of immediate results at the point of care, eliminating the need for sample transportation and laboratory processing. With the growing focus on patient-centered care and the need for quick diagnosis, the demand for rapid test kits is on the rise
- Technological advancements: Rapid test kits are benefiting from advancements in technology, particularly in the fields of immunology, molecular biology, and microfluidics. These advancements have led to improved sensitivity, specificity, and accuracy of the tests, making them more reliable and efficient
- Expanding application areas: Rapid test kits are finding applications in a wide range of healthcare settings, including hospitals, clinics, emergency departments, and even in-home testing. They are also being used in non-clinical settings, such as community screening programs, remote or resource-limited areas, and even for self-testing purposes
- COVID-19 impact: The COVID-19 pandemic has significantly boosted the demand for rapid test kits, especially for the detection of SARS-CoV-2 antigens or antibodies. These tests have played a crucial role in mass testing campaigns, screening efforts, and infection control measures
- Regulatory considerations: As the market for rapid test kits continues to grow, regulatory bodies are focusing on ensuring the quality, accuracy, and reliability of these tests. Harmonization of regulations and the establishment of quality control standards are expected to drive market growth while ensuring patient safety
Global Rapid Test Kit Market Segmentations
Market Breakup by Type
- Rapid Antigen Test
- Rapid Antibody Test
Market Breakup by Product Type
- Over the counter (OTC) Rapid Test Kit
- Professional Rapid Test Kit
Market Breakup by Technology
- Lateral Flow Assays
- Solid Phase
- Agglutination
- Others
Market Breakup by Applications
Infectious Disease
- COVID-19
- Hepatitis
- HIV
- Influenza
- Others
- Glucose Monitoring
- Pregnancy and Fertility
- Toxicology
- Cardiology
- Oncology
- Lipid Profile Testing
- Others
Market Breakup by End User
- Hospitals and Clinics
- Home Care
- Diagnostic Centers
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Rapid Test Kit Market Scenario
The market for rapid test kits has experienced significant growth in recent years, driven by the increasing demand for point-of-care testing and the need for quick and accurate diagnostic solutions. Rapid test kits offer several advantages over traditional laboratory-based testing, including rapid results, portability, and ease of use, making them suitable for a wide range of applications in healthcare settings.The global market for rapid test kits is expanding rapidly, fuelled by the growing prevalence of infectious diseases, rising healthcare expenditure, and advancements in technology. The COVID-19 pandemic has further accelerated the market growth, with rapid test kits playing a critical role in the global response to the pandemic, including mass testing campaigns, screening programs, and surveillance efforts.
Key market players in the rapid test kit industry are focusing on product development and innovation to enhance the accuracy, sensitivity, and specificity of their tests. They are also investing in research and development activities to expand the range of diseases and conditions that can be detected using rapid test kits. Additionally, collaborations and partnerships between manufacturers, healthcare providers, and regulatory bodies are playing a crucial role in driving market growth and ensuring the quality and reliability of these tests.
The market for rapid test kits is expected to continue its upward trajectory in the coming years, driven by the increasing adoption of point-of-care testing, technological advancements, and the need for efficient and cost-effective diagnostic solutions. The expanding application areas, including infectious diseases, cardiac markers, pregnancy testing, and drug testing, are also contributing to the market growth.
Global Rapid Test Kit Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- F. Hoffmann-La Roche AG
- Abbott Laboratories Ltd
- Danaher Corporation
- Becton, Dickinson and Company
- PerkinElmer, Inc
- Siemens Healthineers AG
- Thermo Fischer Scientific, Inc
- Bio-Rad Laboratories, Inc
- OraSure Technologies, Inc
- Biomerieux Inc
- ACON Laboratories, Inc
- Cellex, Inc
- MyMD Pharmaceuticals Inc
- Luminex Corporation
- Eurofins Scientific SE
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche AG
- Abbott Laboratories Ltd
- Danaher Corporation
- Becton, Dickinson and Company
- PerkinElmer, Inc.
- Siemens Healthineers AG
- Thermo Fischer Scientific, Inc.
- Bio-Rad Laboratories, Inc.
- OraSure Technologies, Inc.
- Biomerieux Inc.
- ACON Laboratories, Inc.
- Cellex, Inc.
- MyMD Pharmaceuticals Inc.
- Luminex Corporation
- Eurofins Scientific SE
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | July 2023 |
| Forecast Period | 2023 - 2031 |
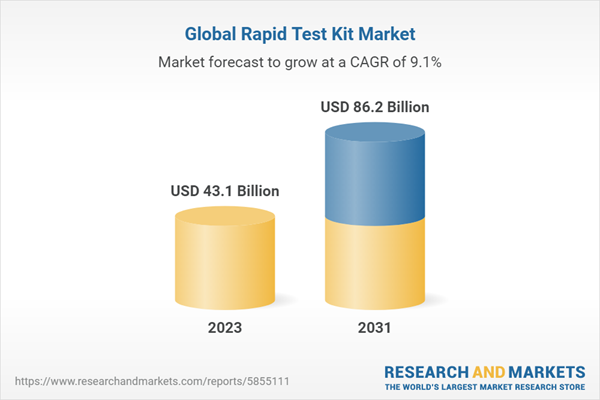
| Estimated Market Value ( USD | $ 43.1 Billion |
| Forecasted Market Value ( USD | $ 86.2 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |