Introduction
Clinical trial biorepository and archiving solutions play a crucial role in the storage, management, and preservation of biological samples and associated data collected during clinical trials. These solutions ensure the integrity and traceability of samples, facilitating research and development of new therapies and treatments. This market introduction provides an overview of the clinical trial biorepository and archiving solutions market and highlights key trends shaping its growth.
The global market for clinical trial biorepository and archiving solutions is witnessing significant growth due to several factors. Firstly, the increasing number of clinical trials and the growing complexity of biomedical research have led to a surge in the volume of biological samples generated. Biorepositories provide the necessary infrastructure and expertise to store and manage these samples, ensuring their long-term preservation for future analysis.
Key Trends in the Clinical Trial Biorepository and Archiving Solutions Market
Key trends in the clinical trial biorepository and archiving solutions market include:
- Adoption of automation and robotics: The integration of automated systems and robotics in biorepositories has gained traction. These technologies streamline sample handling, storage, and retrieval processes, minimizing human errors and improving operational efficiency
- Increasing use of cloud-based storage: Cloud-based storage solutions offer scalability, flexibility, and cost-effectiveness in managing large volumes of data generated from clinical trials. They provide secure and centralized access to data, facilitating collaboration and data sharing among researchers
- Implementation of blockchain technology: Blockchain technology is being explored for enhancing data security, integrity, and traceability in biorepositories. It ensures transparency and immutability of data, providing a reliable and auditable record of sample custody, consent, and data access
Clinical Trial Biorepository and Archiving Solutions Market Segmentations
Market Breakup by Products
- Preclinical Products
- Clinical Products
Market Breakup by Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Market Breakup by Services
- Biorepository Services
- Archiving Services
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Clinical Trial Biorepository and Archiving Solutions Market Overview
The market for clinical trial biorepository and archiving solutions plays a crucial role in supporting biomedical research and drug development. Biorepositories serve as repositories for biological samples collected during clinical trials, storing them in a secure and controlled environment. These samples, including blood, tissue, and other biomaterials, provide valuable resources for researchers to study diseases, develop new therapies, and advance personalized medicine.The demand for clinical trial biorepository and archiving solutions is driven by several factors. First, the increasing complexity of clinical trials and the growing need for precise biomarker analysis require efficient sample management and storage systems. Biorepositories provide the infrastructure and expertise to handle large volumes of samples and ensure their quality and integrity throughout the trial process.
Overall, the market for clinical trial biorepository and archiving solutions is poised for growth as the demand for high-quality samples, data integrity, and regulatory compliance continues to increase. Biorepositories that offer comprehensive solutions, incorporating advanced technologies and ensuring the highest standards of quality and security, will play a crucial role in advancing medical research and therapeutic development.
Clinical Trial Biorepository and Archiving Solutions Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Azenta U.S., Inc
- Thermo Fisher Scientific Inc. (Patheon)
- Precision for Medicine, Inc
- Medpace
- ATCC
- Q2 Solutions
- Labconnect
- Charles River Laboratories
- Cell&Co
- LabCorp Drug Development
Table of Contents
Companies Mentioned
- Azenta U.S., Inc.
- Thermo Fisher Scientific Inc. (Patheon)
- Precision for Medicine, Inc.
- Medpace
- ATCC
- Q2 Solutions
- Labconnect
- Charles River Laboratories
- Cell&Co
- LabCorp Drug Development
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | June 2023 |
| Forecast Period | 2023 - 2031 |
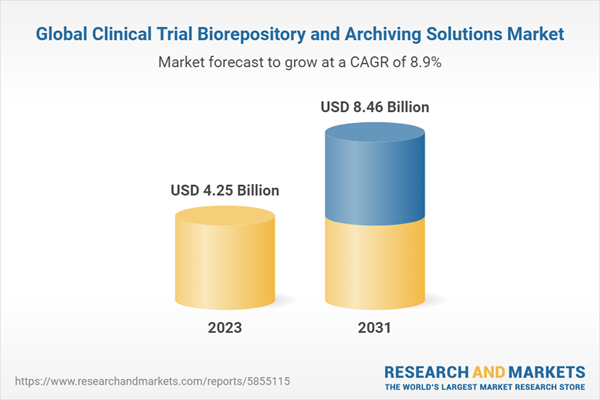
| Estimated Market Value ( USD | $ 4.25 Billion |
| Forecasted Market Value ( USD | $ 8.46 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |