Biosimilar Insulin Glargine and Lispro: Introduction
Biosimilar insulin glargine and lispro are biologic medications that are developed to be highly similar to their respective reference products, insulin glargine and insulin lispro. These biosimilar versions offer a more affordable alternative for the treatment of diabetes, while maintaining similar safety and efficacy profiles.Insulin glargine is a long-acting insulin analog used to control blood sugar levels in patients with diabetes. Biosimilar versions of insulin glargine have been developed to provide a more cost-effective option for patients who require long-acting insulin therapy.
Insulin lispro is a rapid-acting insulin analog that helps to control blood sugar spikes after meals. Biosimilar versions of insulin lispro have been developed to offer a more affordable option for patients who need rapid-acting insulin for effective glycemic control.
Key Trends in the Asia Pacific Biosimilar Insulin Glargine and Lispro Market
In the Asia Pacific region, the market for biosimilar insulin glargine and lispro is witnessing significant growth due to several key trends:- Increasing Diabetes Prevalence: The Asia Pacific region has experienced a significant rise in diabetes prevalence, primarily driven by lifestyle changes, urbanization, and an aging population. Biosimilar insulin glargine and lispro provide more affordable options for diabetes management, addressing the increasing demand for cost-effective treatments
- Government Initiatives and Policies: Governments across the Asia Pacific region are implementing policies and initiatives to promote the adoption of biosimilars, including insulin glargine and lispro, as part of their efforts to improve healthcare accessibility and affordability. These initiatives aim to reduce healthcare costs, increase treatment options, and enhance patient access to essential therapies
- Patent Expirations and Market Entry: The expiration of patents for originator insulin glargine and lispro products has opened the door for biosimilar manufacturers to enter the market. The availability of biosimilars provides competition and increases product choices, driving market growth and potentially lowering treatment costs
- Growing Acceptance and Trust: Healthcare professionals and patients in the Asia Pacific region are gaining confidence in biosimilars, including biosimilar insulin glargine and lispro. Robust regulatory frameworks and rigorous approval processes ensure the safety, efficacy, and quality of biosimilars, contributing to their increased acceptance and trust among healthcare stakeholders
- Cost-Effectiveness and Affordability: Biosimilar insulin glargine and lispro offer cost savings compared to their reference products, making them more affordable for patients and healthcare systems. This affordability factor plays a crucial role in increasing patient access to diabetes treatment and addressing the economic burden of managing diabetes in the region
Asia Pacific Biosimilar Insulin Glargine and Lispro Market Segmentations
Market Breakup by Type
- Branded Drug
- Biosimilar Drug
Market Breakup by Indications
- Type 1 Diabetes
- Type 2 Diabetes
Market Breakup by Distribution Channel
- Hospitals
- Commercial
- Store-Based
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Asia Pacific Biosimilar Insulin Glargine and Lispro Market Scenario
The Asia Pacific market for biosimilar insulin glargine and lispro is a rapidly growing and evolving sector within the healthcare industry. Biosimilar insulin glargine and lispro are emerging as viable alternatives to the reference products, offering similar efficacy and safety profiles at a more affordable price point. This market introduction focuses on the growing demand and adoption of biosimilar insulin glargine and lispro in the Asia Pacific region.The market introduction of biosimilar insulin glargine and lispro is driven by several factors. Firstly, the rising prevalence of diabetes in the Asia Pacific region is a significant driver. As the number of people with diabetes continues to increase, there is a growing need for cost-effective insulin therapies. Biosimilar insulin glargine and lispro provide an affordable option for diabetes management, addressing the increasing demand for accessible and affordable treatments.
Furthermore, government initiatives and policies aimed at promoting the use of biosimilars play a crucial role in market growth. Governments across the Asia Pacific region are implementing strategies to encourage the adoption of biosimilar products, including insulin glargine and lispro. These initiatives focus on reducing healthcare costs, improving treatment access, and enhancing patient outcomes.
Overall, the Asia Pacific market for biosimilar insulin glargine and lispro is characterized by increasing demand, favorable government initiatives, patent expirations, growing acceptance, and regulatory support. These factors contribute to the market's growth and present opportunities for biosimilar manufacturers to meet the healthcare needs of the region.
Asia Pacific Biosimilar Insulin Glargine and Lispro Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Sanofi S.A
- Biocon Limited
- Novo Nordisk A/S
- Wockhardt Ltd
- Eli Lily and Company
- Julphar Diabetes LLC
- SAJA Pharmaceuticals
- Gan & Lee Pharmaceutical Ltd
- Cipla Limited
- Merck & Co
Table of Contents
Companies Mentioned
- Sanofi S.A.
- Biocon Limited
- Novo Nordisk A/S
- Wockhardt Ltd.
- Eli Lily and Company
- Julphar Diabetes LLC
- SAJA Pharmaceuticals
- Gan & Lee Pharmaceutical Ltd.
- Cipla Limited
- Merck & Co.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | June 2023 |
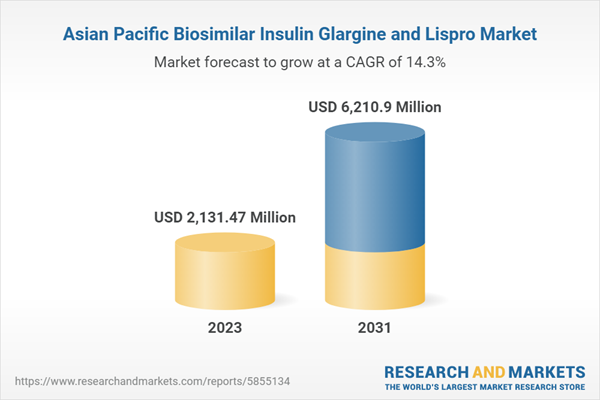
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 2131.47 Million |
| Forecasted Market Value ( USD | $ 6210.9 Million |
| Compound Annual Growth Rate | 14.3% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 10 |