Tracheostomy Products: Introduction
Tracheostomy is a medical surgical procedure which is performed on the patients who are suffering from respiratory issues. The respiratory issues could be due to suffering from any respiratory disorders such as sleep apnea, respiratory infections and major diseases as chronic obstructive pulmonary disease (COPD). In tracheostomy, the healthcare professionals make an opening in the neck which could be temporary or permanent. The equipment used in tracheostomy are known as tracheostomy products such as tracheostomy tube, tracheostomy tube holders, speaking valves, suctioning devices, tracheostomy care kits, among others.Tracheostomies offer multiple benefits and advantages in patient care. They help to maintain a patent airway, deliver oxygen, facilitate mechanical ventilation, and allow for effective suctioning of respiratory secretions. Tracheostomies also minimize the high risk of sophistications related with prolonged intubation, such as damage to the vocal cords and throat, and can improve patient comfort and communication. Tracheostomy management requires specialized care and continuous monitoring. This includes regular tracheostomy tube cleaning, suctioning, and dressing changes, as well as monitoring for complications such as infection, tube blockage, or accidental decannulation.
Global Tracheostomy Products Market Analysis
There are several key trends justifying the global tracheostomy products market growth including the increasing technological advancement in the field. Major key players are focusing more on developing advanced tracheostomy products that are more convenient to use and enhanced versions of current tracheostomy products that are available in the market. One of the major advancements that manufacturers are trying to make is reducing the product size and increasing the efficacy as reduction in product size will directly result in increased patient comfort, increased convenience of healthcare professionals while installations among other benefits.Portable and user-friendly products, such as lightweight and disposable tracheostomy tubes, are gaining popularity to meet the needs of patients and provide convenience to caregivers in non-hospital settings. The rising prevalence of chronic respiratory conditions, such as chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA), is also likely to aid the tracheostomy products market development. The mentioned conditions often require long-term respiratory support such as including tracheostomy procedures which lead to a sustained market growth in tracheostomy products.
Global Tracheostomy Products Market Segmentations
Tracheostomy Products Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup Product Type
- Tracheostomy Tubes
- Ventilation Accessories
- Clean and Care Kits
- Others
Market Breakup by Technique
- Percutaneous Dilatational Tracheostomy
- Surgical Tracheostomy
Market Breakup by End User
- Hospitals
- Ambulatory Care Centers
- Home Care Settings
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Tracheostomy Products Market Overview
Tracheostomy products play an important role and provides many opportunities to the healthcare industry. There are several major factors that are driving the global tracheostomy products market growth including the rising prevalence of respiratory disorders such as sleep apnea, respiratory infections and major diseases as chronic obstructive pulmonary disease (COPD). Tracheostomy products are used to help the patients who are having breathing issues due to suffering from any of the respiratory disease driving the market growth.Additionally, increasing geriatric population is also a major factor as people who are aged are more likely to generate respiratory disorders at this point of age in their lives. Hence, increasing aging population is also a major factor for the growth of the global tracheostomy products market. Along with the above-mentioned factors, there are several other factors which are likely to aid the tracheostomy products market expansion, such as increasing advanced development in the products and their quality. More flexible tracheostomy tubes are now available for the convenience of the patient.
Global Tracheostomy Products Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Medtronic
- Stening SRL
- Bicakcilar Medical
- TROGE MEDICAL GmbH
- Henan Tuoren Medical Device Co. Ltd.
- Fisher & Paykel Healthcare Limited
- Medis Medical Ltd.
- Pulmodyne, Inc.
- Fuji Systems
- DEAS S.R.L.
- Sterimed Group
- Smiths Medical
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Medtronic
- Stening SRL
- Bicakcilar Medical
- TROGE MEDICAL GmbH
- Henan Tuoren Medical Device Co. Ltd.
- Fisher & Paykel Healthcare Limited
- Medis Medical Ltd.
- Pulmodyne, Inc.
- Fuji Systems
- DEAS S.R.L.
- Sterimed Group
- Smiths Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
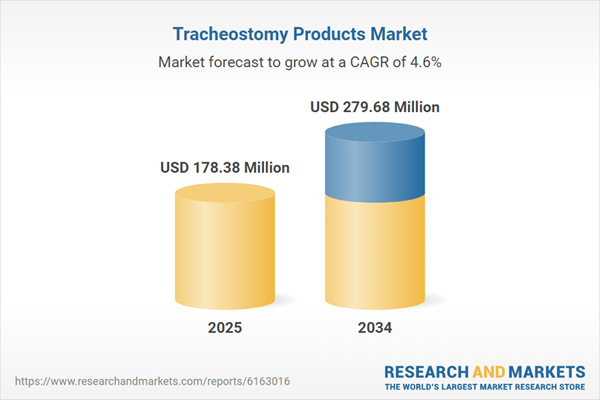
| Estimated Market Value ( USD | $ 178.38 Million |
| Forecasted Market Value ( USD | $ 279.68 Million |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |