Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key trends shaping the market include the rising demand for personalized medicine and biologics, which require sophisticated formulation techniques and specialized handling. Additionally, advancements in nanotechnology, drug delivery systems, and continuous manufacturing are fueling innovation in outsourced formulation development. The growing prevalence of chronic diseases and an aging global population are further amplifying the demand for novel and effective drug formulations. Moreover, increasing investments in emerging markets and favorable government policies supporting pharmaceutical outsourcing are creating new growth avenues.
Despite these positive factors, the market faces challenges such as stringent regulatory requirements, concerns about intellectual property protection, and quality control issues. Variability in outsourcing partner capabilities and geographical risks can also hinder seamless collaboration. Furthermore, supply chain disruptions and delays in clinical trials pose operational risks to formulation development outsourcing projects.
Key Market Drivers
Cost Efficiency and Reduction of Capital Expenditure
Cost efficiency and reduction of capital expenditure are among the primary drivers propelling the growth of the global formulation development outsourcing market. Pharmaceutical companies face increasing pressure to optimize their operational costs while accelerating drug development timelines. Outsourcing formulation development allows these companies to significantly lower upfront investments in infrastructure, equipment, and skilled personnel, which are often costly and time-consuming to establish internally. By leveraging the capabilities of specialized contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs), pharmaceutical firms can avoid heavy capital expenditure associated with setting up and maintaining state-of-the-art laboratories and manufacturing facilities.This strategic shift toward outsourcing enables companies to convert fixed costs into variable costs, providing greater financial flexibility and risk mitigation. Instead of committing large sums to build in-house capabilities that may not be fully utilized at all times, companies pay for services as needed, improving cash flow management. Moreover, outsourcing partners typically possess established, validated processes and regulatory know-how, which can help reduce costly delays and potential compliance issues, further contributing to overall cost savings.
Key Market Challenges
Stringent Regulatory Compliance and Approval Processes
Stringent regulatory compliance and approval processes represent one of the most significant challenges in the global formulation development outsourcing market. The pharmaceutical industry operates under rigorous regulations set by authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regional regulatory bodies. These regulations ensure the safety, efficacy, and quality of drug formulations, but they also impose complex requirements on every stage of formulation development. For companies outsourcing these activities, navigating this regulatory landscape can be particularly demanding.Outsourcing partners must adhere strictly to Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and other quality standards to meet regulatory expectations. Any deviation or non-compliance can lead to severe consequences, including delays in product approvals, costly recalls, or legal penalties. Pharmaceutical companies rely on their CROs and CDMOs to maintain transparency and documentation accuracy throughout the development process to satisfy these stringent criteria.
Additionally, regulatory agencies require comprehensive and robust data submissions covering formulation composition, stability, bioavailability, and manufacturing processes. Coordinating these requirements with external partners often results in complex communication channels and extensive documentation efforts, increasing the risk of errors or omissions. This complexity is further magnified when multiple regulatory jurisdictions are involved, each with unique standards and procedural nuances.
Key Market Trends
Rise of Personalized Medicine and Biologics
The rise of personalized medicine and biologics is a major trend driving the evolution of the formulation development outsourcing market. Personalized medicine focuses on tailoring treatments to individual patients based on their genetic profile, lifestyle, and disease characteristics, moving away from the traditional “one-size-fits-all” approach. This shift demands highly specialized formulation techniques that can accommodate unique drug delivery mechanisms, dosage forms, and stability requirements.Biologics, which include complex molecules such as monoclonal antibodies, vaccines, and gene therapies, represent a rapidly growing segment within pharmaceuticals. These drugs are inherently more sensitive and challenging to formulate compared to conventional small-molecule drugs. Their development requires advanced technology platforms and stringent control over manufacturing conditions to maintain efficacy and safety.
Pharmaceutical companies often lack the in-house capabilities or the necessary infrastructure to efficiently develop and manufacture these complex biologics and personalized therapies. As a result, they increasingly turn to contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) that possess the specialized expertise, technology, and regulatory knowledge needed to manage such complexities.
Outsourcing formulation development in this context helps companies accelerate innovation, reduce development risks, and optimize costs while ensuring high-quality standards. Moreover, these external partners are equipped to handle the stringent regulatory requirements associated with biologics and personalized medicines, facilitating faster approval processes.
Key Market Players
- Charles River Laboratories
- Aizant Drug Research Solutions Private Limited
- Catalent Inc.
- Laboratory Corporation of America Holdings
- Syngene International Ltd.
- Irisys LLC
- Intertek Group PLC
- Piramal Pharma Solutions
- Qiotient Sciences Ltd.
- Patheon Inc.
- Emergent BioSolutions Inc.
- Lonza Group
Report Scope:
In this report, the Global Formulation Development Outsourcing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Formulation Development Outsourcing Market, By Service:
- Preformulation
- Formulation Development
Formulation Development Outsourcing Market, By Formulation:
- Oral
- Injectable
- Topical
- Others
Formulation Development Outsourcing Market, By Therapeutic Area:
- Oncology
- Infectious Disease
- Neurology
- Hematology
- Respiratory
- Cardiovascular
- Dermatology
- Others
Formulation Development Outsourcing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Formulation Development Outsourcing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Charles River Laboratories
- Aizant Drug Research Solutions Private Limited
- Catalent Inc.
- Laboratory Corporation of America Holdings
- Syngene International Ltd.
- Irisys LLC
- Intertek Group PLC
- Piramal Pharma Solutions
- Qiotient Sciences Ltd.
- Patheon Inc.
- Emergent BioSolutions Inc.
- Lonza Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
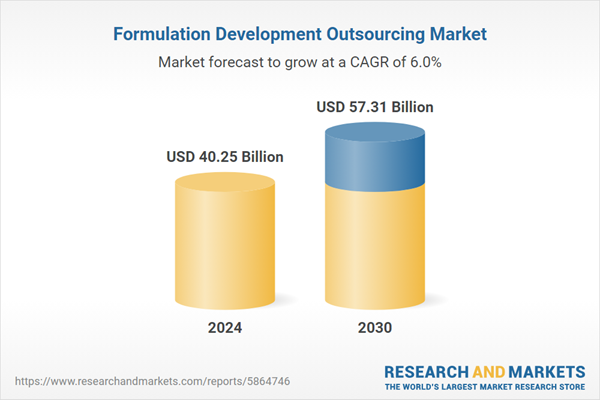
| Estimated Market Value ( USD | $ 40.25 Billion |
| Forecasted Market Value ( USD | $ 57.31 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |