The concentrates of plasma-derived factors are very good at reducing bleeding episodes in hemophiliacs. Consequently, the Plasma-derived Factor Concentrates segment will acquire around 40% share in the market by 2030. Hemophiliacs' risk of developing viral infections can be decreased by using a safe, regulated plasma-derived factor concentrate. Concentrates of plasma-derived factors are easily accessible to a greater number of hemophilia patients due to their ubiquitous availability in numerous areas and nations. They add the necessary clotting component, which improves the effectiveness with which blood clots and stops bleeding.
The major strategies followed by the market participants are Partnerships as the key developmental strategy to keep pace with the changing demands of end users. For instance, In May, 2023, Novo Nordisk A/S teamed up with 2seventy Bio, Inc., to facilitate the creation of vivo gene editing treatment for hemophilia A. Additionally, In December, 2019, Bayer AG signed a partnership with Children's Hospital of Philadelphia (CHOP), to develop non-replacement therapy (NRT) for the treatment of haemophilia A and B.
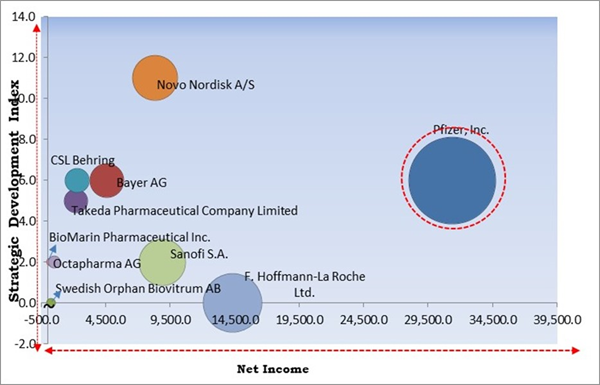
The Cardinal Matrix - Market Competition Analysis
Based on the Analysis presented in the The Cardinal Matrix; Pfizer, Inc. is the forerunner in the Market. In January, 2022, Pfizer signed a research collaboration and license agreement with Dren Bio to concentrate on the discovery and development of therapeutic bispecific antibodies for select oncology targets utilizing Dren Bio’s proprietary Targeted Myeloid Engager and Phagocytosis Platform. Companies such as F. Hoffmann-La Roche Ltd., Sanofi S.A., Novo Nordisk A/S are some of the key innovators in Hemophilia Market.
COVID-19 Impact Analysis
Patients had significant effects as a result of the COVID-19 pandemic's restrictions, including problems accessing healthcare, difficulties with the treatment supply chain, and a fall in market demand. But by January 2021, the market's supply and demand were back to normal. The global COVID-19 pandemic had a negative effect on the market because people with chronic medical disorders, such as hemophilia, needed to take extra steps to reduce their chance of getting the virus. This was mainly because such people were more likely to be exposed to infectious diseases than the general population. As a result, the market grew more slowly during the pandemic.Market Growth Factors
Growing government efforts to expand healthcare infrastructure
To effectively manage the treatment of hemophilia, government officials are introducing new medicines. Therefore, it is probable that a rising number of product approvals will fuel market expansion. The U.S. Food and Drug Administration, for instance, approved Hemgenix (etranacogene dezaparvovec), an adeno-associated gene therapy based on viral vector, in November 2022 for the treatment of people with hemophilia B (congenital Factor IX deficiency). Blood levels of Factor IX are raised by the Factor IX protein, which reduces bleeding. When the COVID-19 pandemic emerged, many governments upped their spending to build healthcare infrastructure. Therefore, as more money is being invested in the healthcare industry, it is accelerating the growth of the market.Increasing modernization of treatment techniques
Opportunities for development are provided by several therapeutic modalities, including recombinant factor replacement therapy, which serves as the first-line therapy for people with moderate to severe hemophilia. Gene therapies also focus on locating the broken DNA bases and replacing them with functional ones; this could create lucrative chances for the market's current players throughout the forecast period. Thus, the management of hemophilia has been transformed by developments in medical technology, including the creation of novel therapeutics and gene therapies. In order to enhance patient outcomes, new products and therapies are constantly being developed, which is fueling market expansion.Market Restraining Factors
High cost of treatment for hemophilia
Health plans currently assess costs as sums of factor units rather than as a function of individual characteristics like age, the use of "on-demand" vs. prophylactic medications, the use of inhibitors, the type of patient, or adherence. Furthermore, they are unable to evaluate patient variability because they are unsure of which members receive on-demand or prophylactic treatment. One of the most dangerous complications of hemophilia is developing inhibitors, which are expensive to cure. Tolerance to factors is a trait of patients who develop inhibitors. Their inhibitor thus prohibits replacement factor from being used effectively. Hence, the high cost of various hemophilia treatments lowers their adoption, which may slow the market's growth.Distribution Channel Outlook
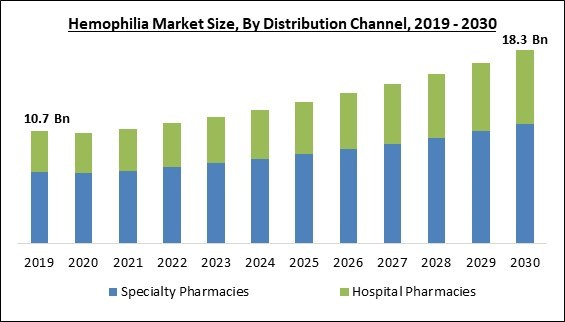
Based on distribution channel, the market is bifurcated into hospital pharmacies and specialty pharmacies. The hospital pharmacies segment witnessed a considerable growth rate in the market in 2022. Growth of this segment is anticipated to be fueled by the approval of gene therapy, which will mostly be used in hospitals because it needs to be continuously monitored for side effects. The majority of clotting factor concentrates, and other hemophilia drugs may often be found in hospital pharmacies. This access is crucial because hemophilia treatments can be complicated, and having a range of medications available enables healthcare professionals to customize therapies to meet the needs of specific patients.Type Outlook
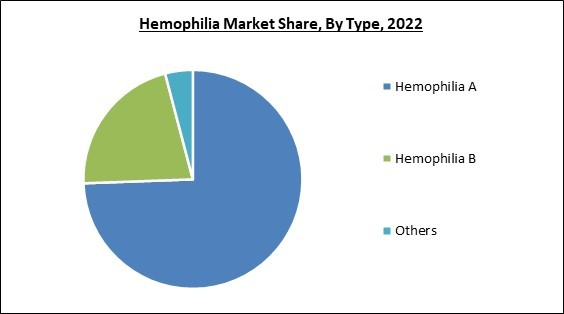
Based on type, the market is divided into hemophilia A, hemophilia B, and others. The hemophilia A segment garnered the highest revenue share in the market in 2022. A genetic condition results in a deficiency of factor VIII, which helps blood clot. The high prevalence of hemophilia A in developed nations and encouraging government activities to introduce products in important markets like the United States, Europe, and Japan are the factors behind the dominance of the segment. In 2021, the United States, India, and Brazil, respectively, had 14098, 21350, and 11141 type A cases, according to the WFH survey.Treatment Type Outlook
On the basis of treatment type, the market is segmented into on-demand, cure, and prophylaxis. The on-demand segment recorded a significant revenue share in the market in 2022. By addressing bleeding episodes as they arise, on-demand treatment makes sure that clotting factor replacement is given just when it is required. The rapid management of acute bleeding episodes can be accomplished with this focused strategy. With on-demand care, patients might forgo repeated infusions when there is no significant bleeding, potentially lightening their treatment load.Therapy Outlook
By therapy, the market is categorized into factor replacement therapy, desmopressin & fibrin sealants, and gene therapy & monoclonal antibodies. The factor replacement therapy segment witnessed the maximum revenue share in the market in 2022. Patients with both type A and type B hemophilia can replace missing clotting factors using factor replacement therapy, which is recognized as a conventional form of treatment. For type A patients, factor VIII replacement products are utilized, while for type B patients, factor IX replacement products are used.Factor Replacement Therapy Outlook
The factor replacement therapy segment is further subdivided into plasma-derived factor concentrates and recombinant factor concentrates. The recombinant factor concentrates segment procured the highest revenue share in the market in 2022. Recombinant factor VIII (rFVIII) products are generating a lot of interest right now because they provide a technological means of extending the half-life of FVIII in treated patients with hemophilia A (HA) and lowering the danger of the development of alloantibodies (inhibitors) against FVIII. Because they are produced using virus inactivation techniques akin to those employed in pd-FVIII concentrates, all rFVIII products have an improved safety profile.Regional Outlook
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region garnered the maximum revenue share in the market in 2022. Key players' presence, increasing patient treatment adoption, advantageous financial assistance, the existence of centers specifically dedicated to treating hemophilia patients, and the disorder's expanding prevalence may all be credited with the regional market's growth. Over the course of the forecast period, it is anticipated that demand for hemophilia medications will increase due to the government's increased efforts to encourage their usage. The utilization of hemophilia in important treatments like replacement therapy, immunotolerance induction therapy, and gene therapy is also a major driver of growth in this industry.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Takeda Pharmaceutical Company Limited, CSL Limited (CSL Behring), Pfizer, Inc., Bayer AG, F. Hoffmann-La Roche Ltd., Novo Nordisk A/S, Octapharma AG, BioMarin Pharmaceutical Inc., Swedish Orphan Biovitrum AB (Investor AB) and Sanofi S.A.
Strategies Deployed in the Market
Partnerships, Collaborations and Agreements:
- May-2023: Novo Nordisk A/S teamed up with 2seventy Bio, Inc., an immuno-oncology cell therapy company, to develop innovative treatment solutions for hemophilia A. The partnership would facilitate the creation of vivo gene editing treatment for hemophilia A.
- Jan-2022: Pfizer signed a research collaboration and license agreement with Dren Bio, a biopharmaceutical company, a developer of therapeutic antibodies for the treatment of cancer. This agreement aimed to concentrate on the discovery and development of therapeutic bispecific antibodies for selecting oncology targets utilizing Dren Bio’s proprietary Targeted Myeloid Engager and Phagocytosis Platform.
Product Launches and Product Expansions:
- May-2022: Takeda Pharmaceutical Company Limited introduced Adynovate, a recombinant Factor VIII treatment for hemophilia. The treatment offers interactive and personalized prophylaxis options. Furthermore, the treatment is viable with the BAXJECT III system.
Mergers & Acquisition:
- Oct-2022: Novo Nordisk A/S acquired Forma Therapeutics, a sickle cell disease treatment specialist. The acquisition strengthens Novo Nordisk’s sickle cell disease offerings.
- Mar-2022: Pfizer, Inc. announced the acquisition of Arena Pharmaceuticals, an immuno-inflammatory disease specialist. The acquisition builds up the company's capability for immuno-inflammatory disease treatment.
Trials and Approvals:
- Jun-2023: BioMarin Pharmaceutical Inc. received approval from the United States Food and Drug Administration (FDA) for its ROCTAVIAN gene therapy for patients with severe cases of hemophilia A.
- Mar-2023: The Ministry of Food and Drug Safety approved Takeda Korea's Obizur, a recombinant product that is used for controlling blood clots in patients.
- Feb-2023: The U.S. Food and Drug Administration (FDA) approved Sanofi's ALTUVIIIO, a recombinant protein used for controlling bleeding in patients with hemophilia A.
- Nov-2022: CSL Behring received approval from The US Food and Drug Administration (FDA) for its Hemgenix gene therapy for Hemophilia treatment.
Scope of the Study
By Distribution Channel
- Specialty Pharmacies
- Hospital Pharmacies
By Type
- Hemophilia A
- Hemophilia B
- Others
By Treatment Type
- Prophylaxis
- On-demand
- Cure
By Therapy
- Factor Replacement Therapy
- Recombinant Factor Concentrates
- Plasma-derived Factor Concentrates
- Desmopressin & Fibrin Sealants
- Gene Therapy & Monoclonal Antibodies
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Takeda Pharmaceutical Company Limited
- CSL Limited (CSL Behring)
- Pfizer, Inc.
- Bayer AG
- F.Hoffmann-La Roche Ltd.
- Novo Nordisk A/S
- Octapharma AG
- BioMarin Pharmaceutical Inc.
- Swedish Orphan Biovitrum AB (Investor AB)
- Sanofi S.A.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Takeda Pharmaceutical Company Limited
- CSL Limited (CSL Behring)
- Pfizer, Inc.
- Bayer AG
- F. Hoffmann-La Roche Ltd.
- Novo Nordisk A/S
- Octapharma AG
- BioMarin Pharmaceutical Inc.
- Swedish Orphan Biovitrum AB (Investor AB)
- Sanofi S.A.