NEXT GENERATION DRUG CONJUGATES MARKET: GROWTH AND TRENDS
Due to their non-immunogenic characteristics, structural reliability, improved clinical profiles, precise payload delivery, reduced side effects, permeability, and enhanced cellular penetration, next-generation drug conjugates have become promising targeted treatments for rare disease conditions, including various cancers. Similar to antibody drug conjugates (ADCs), these advanced forms exhibit enhanced clinical effectiveness and stability, utilizing a variety of non-antibody targeting agents including peptides, amino sugars, lipids, and small molecules.They employ payloads, such as oligonucleotides, antisense oligonucleotides, si-RNA, drugs, and radionuclides for targeted delivery instead of conventional drugs. The combination of targeting ligands and payloads has led to the development of numerous next generation drug conjugates, such as peptide drug conjugates, peptide receptor radionuclide therapy, GalNac conjugates, and si-RNA conjugates, all demonstrating efficacy against conditions like solid tumors, metabolic disorders, and hematological disorders.
The USFDA has authorized six next-generation drug conjugates for treatment purposes, which are Lutathera®, Pluvicto®, Givlaari®, Oxlumo®, Leqvio®, and Amvuttra®. These approvals highlight the clinical achievements of these advanced treatments and their promise for various medical conditions. Through continuous innovation, favorable trial results, accelerated approvals, and joint initiatives, the market for next-generation drug conjugates expects substantial expansion during the projected timeframe.
NEXT GENERATION DRUG CONJUGATES MARKET: KEY INSIGHTS
The report delves into the current state of the next generation drug conjugates market and identifies potential growth opportunities within the industry. Some key findings from the report include:- The pipeline features more than 200 next generation drug conjugates with small molecules, peptides, lipids, amino sugars and virus like particles as the targeting ligands for therapeutic purposes.
- Close to 60% of the next generation drug conjugates use peptides as targeting ligand; of these, majority are being evaluated in clinical trials for the treatment of oncological disorders.
- Over 260 clinical trials have been registered in the past few years to evaluate the safety and efficacy of various next generation drug conjugates; majority of these studies have been conducted across sites in the US.
- Over the past few years, partnership activity in this field has increased for the development of effective drug conjugate therapies.
- Lack of clinical efficacy has been the common reason for drug failure, accounting for discontinuation of more than 55% clinical trials, while several non-drug related issues caused the failure of more than 30% trials.
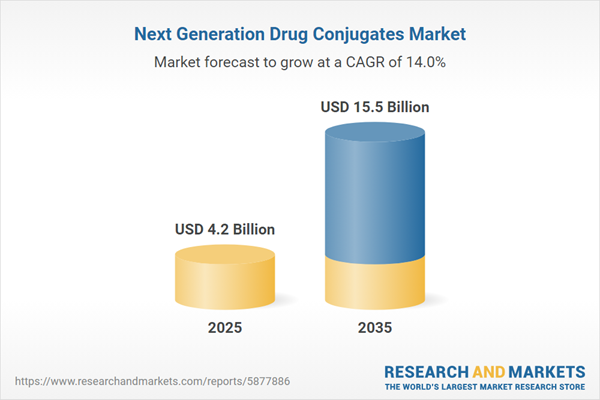
- With growing focus on the development pipeline and encouraging clinical results, the market is anticipated to witness an annualized growth rate of 14.0%, during the next decade.
- The projected opportunity is expected to be well distributed across different routes of administration, target indications and geographical regions.
NEXT GENERATION DRUG CONJUGATES MARKET: KEY SEGMENTS
Currently, Ligand Mediated RNAi Conjugate Occupies the Largest Share of the Next Generation Drug Conjugates Market
In terms of the type of next generation drug conjugates, the market is segmented into ligand mediated RNAi conjugate, peptide-radionuclide conjugate, ligand conjugated anti sense medicine and peptide drug conjugate. Currently, ligand-mediated RNAi conjugates dominate the next-generation drug conjugates market. It is important to note that the upcoming generation of drug conjugates for ligand-attached anti-sense therapies is expected to grow at a higher CAGR.Amino Sugar is Likely to Dominate the Next Generation Drug Conjugates Market in the Coming Decade
In terms of targeting ligand, the market is segmented into amino sugar, lipids and peptide. Currently, peptide accounts for the largest share in the next generation drug conjugates market. In the coming years, the next generation drug conjugates market is expected to be propelled by amino sugar.Currently, Radionuclide Segment Occupies the Largest Share of the Next Generation Drug Conjugates
In terms of payload, the market is segmented into Si-RNA, radionuclide, antisense oligonucleotide and drug. Currently, radionuclides dominate the market for next-generation drug conjugates. It is important to note that the next generation drug conjugates for Si-RNA are expected to expand at a comparatively higher CAGR.Combination Therapies are Likely to Dominate the Next Generation Drug Conjugates Market During the Forecast Period
In terms of type of therapy, the market is segmented into monotherapy and combination therapy. Currently, combination therapy maintains the largest share in the next generation drug conjugates market. This pattern is not expected to alter in the next few decades.Currently, Intravenous Route Occupies the Largest Share of the Next Generation Drug Conjugates Market
In terms of route of administration, the market is segmented into intravenous and subcutaneous. It is worth highlighting that majority of the current next generation drug conjugates market is captured by the intravenous route. However, the next generation drug conjugates market for subcutaneous route is likely grow at a relatively higher CAGR in the coming decade.Hemophilia Segment is the Fastest Growing Segment of the Next Generation Drug Conjugates Market During the Forecast Period
In terms of key target disease indication, the market is segmented into gastroenteropancreatic neuroendocrine tumors, prostate cancer, leptomeningeal carcinomatosis caused by breast cancer brain metastases, hereditary transthyretin amyloidosis, atherosclerotic cardiovascular diseases, severe hypertriglyceridemia, hereditary angioedema, acute hepatic porphyria, primary hyperoxaluria, heterozygous familial hypercholesterolemia, hemophilia, low-risk myelodysplastic syndrome, myelofibrosis, alpha-1 antitrypsin deficiency liver disease and familial chylomicronemia syndrome. It is worth highlighting that, at present, prostate cancer holds larger share in the next generation drug conjugates market. However, the next generation drug conjugates market for hemophilia is likely to grow at a relatively higher CAGR.North America Accounts for the Largest Share of the Market
In terms of key geographical regions, the market is segmented into North America, Europe, and Asia-Pacific and Rest of the World. Majority share is expected to be captured by players based in North America. It is worth highlighting that, over the years, the market in Asia-Pacific and Rest of the World is expected to grow at a higher CAGR.Example Players in the Next Generation Drug Conjugates Market
- Advanced Accelerator Applications (Acquired by Novartis)
- Alnylam Pharmaceuticals
- Arrowhead Pharmaceuticals
- Dicerna Pharmaceuticals
- Geron Corporation
- Ionis Pharmaceuticals
NEXT GENERATION DRUG CONJUGATES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the next generation drug conjugates market, focusing on key market segments, including [A] type of targeting ligand, [B] type of payload, [C] type of therapy, [D] route of administration, [E] target disease indication and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of next generation drug conjugates / next generation targeted therapeutics that are either approved or being evaluated in different stages of development, considering various parameters, such as [A] type of conjugate, [B] type of targeting ligand, [C] type of payload, [D] type of biological target, [E] mechanism of action, [F] stage of development, [G] phase of development, [H] type of therapy, [I] route of administration, [J] target disease indication, [K] therapeutic area and [L] target population. Further, the chapter includes information on various next generation drug conjugate developers, based on their [M] year of establishment, [N] company size, [O] location of headquarters and [P] most active players (in terms of number of drug candidates).
- Company Profiles: In-depth profiles of key industry players involved in the development of next generation drug conjugates, focusing on [A] company overviews, [B] product portfolio, [C] detailed information on the drug candidates which are either approved or are in phase III of clinical development, [D] recent developments and [E] an informed future outlook.
- Clinical Trials: Examination of completed, ongoing, and planned clinical studies of various drug conjugates based on parameters like [A] trial registration year, [B] trial phase, [C] trial status, [D] enrolled patient population, [E] type of sponsor, [F] age group, [G] most active industry players, [H] leading drug candidate, [I] primary purpose, [J] therapeutic area and [K] key geographical regions.
- Partnerships and Collaborations: An analysis of partnerships established in this sector, covering acquisitions, research and development agreements, product development and commercialization agreements, platform / technology licensing agreement, service agreement, clinical trial agreement, joint venture and others.
- Academic Grant Analysis: A comprehensive evaluation of various grants that have been awarded to research institutes engaged in conducting research related to next generation drug conjugates, since 2018, based on various important parameters, such as [A] year of grant award, [B] amount awarded, [C] funding institute center, [D] support period, [E] type of grant application, [F] purpose of grant award, [G] activity code, [H] study section involved, [I] popular NIH departments (based on number of grants awarded), [J] prominent program officers, [K] leading recipient organizations and [L] key regions.
- Publication Analysis: An in-depth analysis of scientific articles focused on next generation drug conjugates based on [A] year of publication, [B] type of publication, [C] type of conjugate, [D] target indication, [C] copyright holders, [D] focus area, [E] leading publishers and [F] key journals (in terms of number of articles published and impact factor).
- Drug Failure Analysis: An in-depth analysis of next generation drug conjugates that failed to progress to later stages of development, based on various relevant parameters, such as trial status of discontinuation, trial phase of discontinuation, average trial year, type of therapy, target indication and reason for drug failure.
- Success Protocol Analysis: An insightful success protocol analysis of recently approved and commercialized next generation drug conjugates, based on several relevant parameters, such as dosing frequency, drug efficacy, drug exclusivity, drug designation, fatality rate, geographical reach, intra-class competition, line of treatment, prevalence, price, type of therapy, and existing competition among developers.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What are the factors that are likely to influence the evolution of this market?
- What is current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with the Research Team
- Free Updated report if the report is 6-12 months old or older
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 48Hour Discovery
- Ablaze Pharmaceuticals
- Advanced Accelerator Applications (a Novartis Company)
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Antios Therapeutics
- Arbutus Biopharma
- ArisGlobal
- Aro Biotherapeutics
- Arrowhead Pharmaceuticals
- AstraZeneca
- Asymchem
- Aura Biosciences
- Bicycle Therapeutics
- Boehringer Ingelheim
- CAMP4 Therapeutics
- Captario
- Cardinal Health
- Case Western Reserve University
- Cellectar Biosciences
- Cerveau Technologies
- CIC bioGUNE
- Clarity Pharmaceuticals
- Clearside Biomedical
- Coherent Biopharma
- Crescendo Biologics
- Cybrexa Therapeutics
- Dicerna Pharmaceuticals
- DTx Pharma
- Eli Lilly
- Empirico
- Entrada Therapeutics
- Esperance Pharmaceuticals
- Eubulus Biotherapeutics
- Evergreen Theragnostics
- Exelixis
- Flamingo Therapeutics
- Genentech
- Genuity Science
- Geron
- GSK
- Gubra
- Hansoh Pharmaceutical
- Horizon Therapeutics
- Idaho State University
- Ionis Pharmaceuticals
- Janssen
- Jiangsu Hansoh Pharmaceutical (a subsidiary of Hansoh Pharmaceutical)
- Kings College Hospital
- LegoChem Biosciences
- Mainline Biosciences
- Mallinckrodt Pharmaceuticals
- Medicines Manufacturing Innovation Centre (MMIC)
- Medison Pharma
- Merck
- Metagenomi
- Myotonic Dystrophy Clinical Research Network (DMCRN)
- National Cancer Institute
- Nimble Therapeutics
- n-Lorem Foundation
- NorthStar Medical Radioisotopes
- Novartis
- Novo Nordisk
- OliX Pharmaceuticals
- Ono Pharmaceutical
- Orano Med
- Oregon Health & Science University
- Orsini Specialty Pharmacy
- Osteros Biomedica
- Ousia Pharma
- Owlstone Medical
- Paradigm4
- PepGen
- Pepscan (acquired by Biosynth)
- PeptiDream
- Pharmaron
- Qilu Pharmaceutical
- Queen Elizabeth University Hospital Birmingham (UHB)
- RayzeBio
- Regeneron Pharmaceuticals
- Roche
- Roivant Sciences
- Royalty Pharma
- Sarepta Therapeutics
- Sharp
- Shenzhen Ascentawits Pharmaceuticals
- Shionogi
- Silence Therapeutics
- Sirnaomics
- Soricimed Biopharma
- Suzhou Ribo Life Science
- taiba rare
- Takeda
- University of Melbourne
- Vaccitech
- Vertex Pharmaceuticals
- Vivo Capital
- Wave Life Sciences
- WuXi STA
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | December 2025 |
| Forecast Period | 2025 - 2035 |
| Estimated Market Value ( USD | $ 4.2 Billion |
| Forecasted Market Value ( USD | $ 15.5 Billion |
| Compound Annual Growth Rate | 14.0% |
| Regions Covered | Global |