Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Pioneering Clinical Success of Viral Vector Production
The clinical success of gene therapies leveraging viral vectors has played a critical role in driving market growth. Breakthroughs such as Luxturna, approved by the FDA for treating Leber congenital amaurosis (LCA), and Zolgensma for spinal muscular atrophy (SMA), underscore the transformative potential of viral vector-based treatments. Zolgensma alone has treated over 3,000 patients globally as of 2023. Additionally, promising advancements in hemophilia B treatments, utilizing AAV vectors to restore clotting factor IX levels, highlight the clinical viability of these delivery tools. Lentiviral vectors have also been widely adopted in CAR-T cell therapies targeting blood cancers. These successes demonstrate the therapeutic efficacy, safety, and long-term benefits of viral vectors, prompting increased investment and adoption across both academic and commercial sectors.Key Market Challenges
Scalability and Commercialization
Scaling up viral vector production for commercial use poses significant operational and financial hurdles. Transitioning from lab-scale to large-scale manufacturing requires overcoming challenges such as maintaining yield consistency, optimizing cell culture conditions, and controlling vector integrity under scaled processes. High-yield production must address issues related to oxygen transfer, nutrient delivery, and shear stress in bioreactors. Additionally, ensuring quality and stability of viral vectors during purification and fill-finish steps is essential but complex. Building compliant manufacturing facilities requires large capital investment and adherence to strict regulatory standards. These scale-related constraints delay commercialization timelines, increase production costs, and limit patient access to advanced therapies, especially when demand accelerates.Key Market Trends
Manufacturing Process Optimization
Process optimization is a critical trend shaping the viral vector production landscape. Industry players are focusing on increasing yield, consistency, and scalability through innovations in upstream and downstream workflows. Efforts include enhancing cell line productivity, refining transfection protocols, and automating quality control. Manufacturers are standardizing production processes to ensure reproducibility across facilities, which aids in regulatory compliance and technology transfer. Moreover, improvements in purification and fill-finish steps are helping reduce impurities and boost product quality. Environmentally sustainable manufacturing approaches - such as reducing waste and energy usage - are also gaining prominence. These optimizations not only lower production costs but also ensure that therapies reach patients more efficiently and reliably.Key Market Players
- Merck KGaA
- FUJIFILM Diosynth Biotechnologies U.S.A
- Cobra Biologics Ltd.
- Thermo Fisher Scientific Inc.
- Waisman Biomanufacturing
- Genezen Laboratories
- Advanced BioScience Laboratories, Inc. (ABL Inc.)
- Novasep Holding S.A.S.
- Orgenesis Biotech Israel Ltd (formerly ATVIO Biotech Ltd.)
- Takara Bio Inc.
Report Scope:
In this report, the Global Viral Vector Production Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Viral Vector Production Market, By Vector Type:
- Adenovirus
- AAV
- Lentivirus
- Retrovirus
- Others
Viral Vector Production Market, By Workflow:
- Upstream Processing
- Vector Amplification and Expansion
- Vector Recovery/Harvesting
- Downstream Processing
- Purification
- Fill Finish
Viral Vector Production Market, By Application:
- Gene and Cell Therapy Development
- Vaccine Development
- Biopharmaceutical and Pharmaceutical Discovery
- Biomedical Research
Viral Vector Production Market, By End User:
- Pharmaceutical and Biopharmaceutical Companies
- Research Institutes
Viral Vector Production Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Viral Vector Production Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company’s specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Merck KGaA
- FUJIFILM Diosynth Biotechnologies U.S.A
- Cobra Biologics Ltd.
- Thermofisher Scientific Inc.
- Waisman Biomanufacturing
- Genezen Laboratories
- Advanced BioScience Laboratories, Inc. (ABL inc.)
- Novasep Holding s.a.s.
- Orgenesis Biotech Israel Ltd (formerly ATVIO Biotech ltd.)
- Takara Bio Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | June 2025 |
| Forecast Period | 2024 - 2030 |
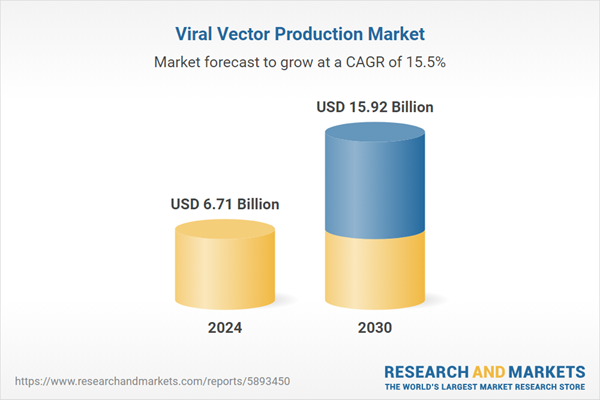
| Estimated Market Value ( USD | $ 6.71 Billion |
| Forecasted Market Value ( USD | $ 15.92 Billion |
| Compound Annual Growth Rate | 15.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |