Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces a significant hurdle due to the frequently asymptomatic nature of chlamydia, which reduces the urgency for patients to seek medical advice. Since many infected individuals show no clinical symptoms, they often go undiagnosed and untreated, which limits the total number of diagnostic tests conducted each year. This situation creates a reliance on proactive public health programs rather than symptomatic patient visits, thereby restricting market growth in areas where routine screening is not rigorously enforced.
Market Drivers
The rising global incidence of Chlamydia trachomatis infections serves as a major market driver, requiring strong surveillance to control the spread of this widespread bacterium. As transmission continues, healthcare systems are emphasizing broad screening to detect untreated individuals and prevent complications like infertility, fueling a steady demand for high-volume laboratory testing. According to a March 2025 MedTech Dive article titled "FDA OKs first at-home test for 3 STIs," CDC data revealed that over 2.4 million cases of syphilis, gonorrhea, and chlamydia were reported in the U.S. in 2023, reinforcing the necessity for ongoing monitoring. Similarly, the UK Health Security Agency’s December 2025 report noted that 601,295 chlamydia tests were conducted on young women aged 15 to 24 in England during 2024, demonstrating the vast scale of national screening efforts.Additionally, the growth of rapid Point-of-Care (POC) technologies and the increasing demand for at-home testing kits are transforming the market by decentralizing care access. These innovations overcome barriers regarding privacy and convenience, prompting individuals who might avoid clinics to seek diagnosis, while regulatory agencies are increasingly validating these solutions for wider use. As reported by CIDRAP in March 2025, the U.S. FDA authorized the first over-the-counter, at-home test from Visby Medical that detects chlamydia, gonorrhea, and trichomoniasis. This move toward user-initiated testing improves detection rates by identifying asymptomatic cases often missed by traditional clinical methods.
Market Challenges
A major obstacle hindering the Global Chlamydia Infection Diagnostics Market is the often asymptomatic nature of the infection, which discourages voluntary testing. Since most infected individuals lack noticeable symptoms, they feel little immediate motivation to seek medical care or undergo screening. This lack of urgency significantly lowers the volume of patient-initiated visits, making the market heavily reliant on government-funded screening programs rather than organic consumer demand. Consequently, when public health resources are limited or screening mandates are not strictly enforced, testing numbers fall sharply, directly restricting the revenue potential for diagnostic manufacturers.Recent surveillance data confirms this trend, showing that decreased testing activity leads to lower detection rates. For example, the UK Health Security Agency reported in 2025 that chlamydia diagnoses dropped by 13 percent in 2024 compared to the prior year, a decline largely driven by reduced testing volumes among young adults. This contraction illustrates how the lack of clinical symptoms allows infections to remain undetected, effectively reducing the addressable market for diagnostic testing solutions.
Market Trends
The widespread adoption of Multiplex Nucleic Acid Amplification Assays is reshaping diagnostic workflows by allowing the simultaneous detection of Chlamydia trachomatis and co-infections like Neisseria gonorrhoeae and Trichomonas vaginalis. This trend meets the clinical need for comprehensive sexual health profiling, as identifying multiple pathogens in a single test enhances patient management and laboratory efficiency compared to single-target tests. In its "Financial Results for Fourth Quarter of Fiscal 2025" released in November 2025, Hologic, Inc. reported a 1.2 percent increase in molecular diagnostics revenue, a growth attributed to the rising use of their advanced multiplex panels, such as the BV CV/TV assays, which consolidate testing for vaginitis and sexually transmitted pathogens on automated platforms.Concurrently, the rise of decentralized pharmacy-based screening models is redefining patient access by bringing diagnostic services into community retail environments, thereby eliminating obstacles associated with traditional clinical visits. Public health programs are increasingly leveraging local pharmacies to distribute self-collection kits and facilitate immediate intervention, effectively reaching asymptomatic populations who might otherwise skip routine medical appointments. For instance, in February 2025, Surrey County Council formalized a service specification in its "Chlamydia Treatment and Screening 2025-28" agreement, requiring community pharmacies to actively provide chlamydia screening kits to sexually active individuals under 25, highlighting the strategic pivot toward pharmacy-led infrastructure in managing infection rates.
Key Players Profiled in the Chlamydia Infection Diagnostics Market
- F. Hoffmann-La Roche Ltd.
- Abbott Laboratories
- Hologic, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Quidel Corporation
- DiaSorin SpA
- AstraZeneca PLC
- Teva Pharmaceutical Industries Limited
- bioMérieux SA
Report Scope
In this report, the Global Chlamydia Infection Diagnostics Market has been segmented into the following categories:Chlamydia Infection Diagnostics Market, by Test Type:
- Culture Test
- Nucleic Acid Amplification Test (NAAT)
- Direct Fluorescent Antibody Test
- Serology Test and Other
Chlamydia Infection Diagnostics Market, by Type of Infections:
- Genital Chlamydia Infection
- Rectal Chlamydia Infection
- Ocular Chlamydia Infection
Chlamydia Infection Diagnostics Market, by End User:
- Hospitals
- Specialty Clinics
- Diagnostics Centre
Chlamydia Infection Diagnostics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Chlamydia Infection Diagnostics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Chlamydia Infection Diagnostics market report include:- F. Hoffmann-La Roche Ltd.
- Abbott Laboratories
- Hologic, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc
- Quidel Corporation
- DiaSorin SpA
- AstraZeneca PLC
- Teva Pharmaceutical Industries Limited
- bioMérieux SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
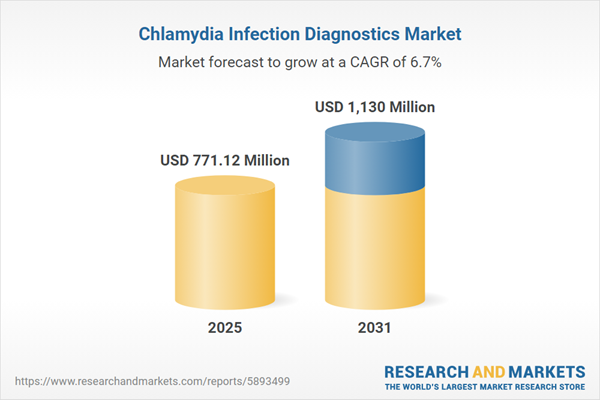
| Estimated Market Value ( USD | $ 771.12 Million |
| Forecasted Market Value ( USD | $ 1130 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |