Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The success of immunotherapies, including immune checkpoint inhibitors and CAR-T cell therapies, has generated interest and confidence in the potential of cancer vaccines. These advancements have highlighted the role of the immune system in targeting cancer cells, driving further research and investment in cancer vaccines. Advances in genomics, proteomics, and bioinformatics have enabled a deeper understanding of tumor biology and the identification of potential vaccine targets. These technological innovations have accelerated the discovery and development of cancer vaccines.
The concept of combining different treatment modalities, such as vaccines with immune checkpoint inhibitors or chemotherapy, has gained traction. Combination therapies have the potential to enhance treatment efficacy and overcome resistance mechanisms. Various global health organizations and initiatives have highlighted the importance of cancer prevention and treatment. These initiatives contribute to increased awareness and funding for cancer vaccine research and development.
Key Market Drivers
Growing Demand of Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have become a cornerstone in modern cancer immunotherapy, offering a transformative approach to treating malignancies that were once considered difficult to manage. These drugs work by targeting checkpoint proteins such as PD-1, PD-L1, and CTLA-4, which act as regulatory switches on immune cells. By blocking these proteins, immune checkpoint inhibitors restore the immune system’s ability to detect and destroy cancer cells. According to a 2024 study published in Nature Reviews Drug Discovery, over 40 FDA-approved indications now exist for checkpoint inhibitors, covering more than 20 cancer types. This rising number of approvals demonstrates the expanding clinical utility and acceptance of these therapies.One of the most compelling aspects of immune checkpoint inhibitors is their ability to produce durable and, in some cases, complete responses in patients with advanced-stage cancers. Recent data from the American Association for Cancer Research (AACR) reveals that five-year survival rates for patients with metastatic melanoma have improved from under 10% to nearly 35% with checkpoint inhibitor therapy. These outcomes are especially significant for patients who had exhausted conventional treatment options. The success stories from checkpoint inhibitor therapies have intensified the focus on immunotherapeutic approaches, including cancer vaccines, which can potentially synergize with these agents to produce even more powerful immune responses.
Checkpoint inhibitors are increasingly being used in combination therapy regimens to overcome resistance mechanisms and broaden their efficacy. Clinical trials are currently exploring over 1,000 combination strategies globally, many of which involve pairing checkpoint inhibitors with cancer vaccines. The rationale behind such combinations lies in their complementary mechanisms - vaccines prime the immune system to recognize tumor-specific antigens, while checkpoint inhibitors unleash T cells to eliminate the identified cancer cells. This integrated approach is driving deeper and more sustained responses, particularly in tumors with low immunogenicity that may not respond well to monotherapies.
The growing demand for immune checkpoint inhibitors is not only driving innovation in therapeutic strategies but also reinforcing the role of cancer vaccines as a critical component of immuno-oncology pipelines. As researchers strive to enhance treatment precision and personalization, vaccines are being developed to target neoantigens identified through next-generation sequencing. The success and continued development of checkpoint inhibitors have built a robust foundation of scientific, clinical, and commercial support for immunotherapies. This momentum is expected to further accelerate interest and investment in cancer vaccines, which are increasingly seen as essential components of comprehensive cancer immunotherapy regimens.
Key Market Challenges
Complexity of Cancer Immunology
Cancer immunology involves the intricate interplay between cancer cells and the immune system, and understanding and manipulating this interaction for therapeutic purposes is no small task. Cancers are highly heterogeneous, meaning that they can have diverse populations of cells with distinct genetic and antigenic profiles. Identifying the right antigens to target with a vaccine becomes challenging, as a one-size-fits-all approach may not be effective. Cancer cells can develop mechanisms to evade immune detection and attack. They can downregulate antigens, express inhibitory molecules, or create an immunosuppressive microenvironment. Developing vaccines that overcome these strategies is complex.Selecting the most appropriate antigens for targeting is a challenge. Not all tumor antigens are equally effective at inducing a strong immune response, and the wrong choice can result in inadequate therapeutic outcomes. The immune system is designed to avoid attacking healthy cells. Overcoming immune tolerance mechanisms while avoiding autoimmune reactions is a delicate balance that must be considered in vaccine design. Ensuring that the vaccine itself is immunogenic and can stimulate a robust immune response is crucial. Some tumors may have a suppressive effect on the immune system, making it difficult to generate a response. Identifying reliable biomarkers that predict which patients will respond positively to a cancer vaccine is a challenge. Responders and non-responders can have varied immune profiles, and finding consistent predictive markers can be difficult.
Key Market Trends
Collaborations and Partnerships
The complex nature of cancer research, vaccine development, and clinical trials often necessitates collaboration among various stakeholders to accelerate progress, share expertise, and pool resources. Developing effective cancer vaccines requires expertise in various fields, including immunology, oncology, virology, genetics, and more. Collaborations allow researchers and organizations to bring together experts from different disciplines to tackle complex challenges. Collaborations enable the sharing of resources, such as research facilities, laboratories, equipment, and reagents. This can reduce costs and accelerate the research and development process. Partnerships provide access to cutting-edge technologies and platforms that individual organizations might not have. This can streamline vaccine development and improve research capabilities.In-depth understanding of cancer biology and immunology requires access to vast amounts of data. Collaborations allow for data sharing, analysis, and integration, facilitating better insights into vaccine targets and mechanisms. Running clinical trials for cancer vaccines often requires collaboration among multiple institutions and hospitals. Partnerships can facilitate patient recruitment, trial logistics, and data collection. Collaborations can attract funding from various sources, including government agencies, private investors, philanthropic organizations, and venture capital firms. This financial support can drive research and development efforts. Partnerships with pharmaceutical companies can help bring cancer vaccines to market more effectively, leveraging established distribution channels, sales teams, and marketing resources.
Key Market Players
- Merck & Co., Inc.
- GSK plc
- Dendreon Pharmaceuticals LLC.
- Dynavax Technologies.
- Ferring B.V.
- Amgen, Inc.
- Moderna, Inc.
- Sanofi SA
- AstraZeneca Pharmaceuticals LP
- Bristol-Myers Squibb Company
Report Scope:
In this report, the Global Cancer Vaccine Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Cancer Vaccine Market, By Indication Type:
- Prostate Cancer
- Melanoma
- Bladder Cancer
- Cervical Cancer
Cancer Vaccine Market, By Vaccine Type:
- Preventive Cancer Vaccines
- Therapeutic Cancer Vaccines
Cancer Vaccine Market, By Technology Type:
- Recombinant Cancer Vaccines
- Whole-Cell Cancer Vaccines
- Viral Vector and DNA Cancer Vaccines
Cancer Vaccine Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cancer Vaccine Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Merck & Co., Inc.
- GSK plc
- Dendreon Pharmaceuticals LLC.
- Dynavax Technologies.
- Ferring B.V.
- Amgen, Inc.
- Moderna, Inc.
- Sanofi SA
- AstraZeneca Pharmaceuticals LP
- Bristol-Myers Squibb Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
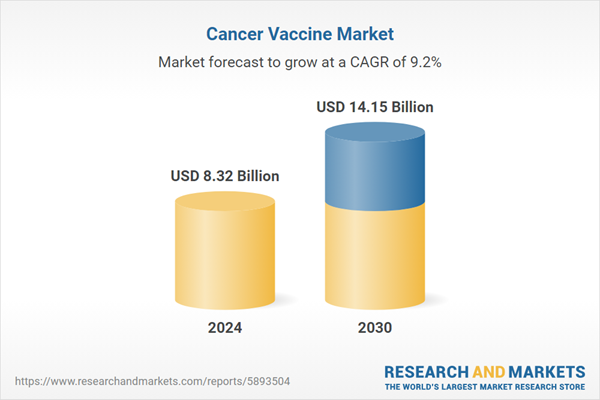
| Estimated Market Value ( USD | $ 8.32 Billion |
| Forecasted Market Value ( USD | $ 14.15 Billion |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |