Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Acute care syndromic testing is especially important in situations where patients present with symptoms that could be attributed to various infectious agents. This testing strategy allows healthcare providers to make timely and informed decisions regarding patient care, isolation measures, and treatment options. It can be particularly valuable during disease outbreaks or pandemics, as it helps healthcare systems efficiently manage and respond to a large number of patients with similar symptoms. However, The specific types of tests used in acute care syndromic testing may include molecular assays (like polymerase chain reaction or PCR tests), rapid antigen tests, serological tests (looking for antibodies), and other diagnostic methods. The choice of tests depends on the suspected pathogens and the clinical context.
Key Market Drivers
Rise in Infectious Disease Outbreaks
The rise in infectious disease outbreaks has a significant impact on driving the growth of the global acute care syndromic testing market. Infectious disease outbreaks create a heightened demand for rapid and accurate diagnostic solutions, and acute care syndromic testing addresses this need. Influenza is the most common infectious disease in the world, but some diseases are more prevalent than others. In the United States alone, about 1 in every 5 people gets the flu every year, but only about 300 people get prion disease each year. Acute care syndromic testing enables healthcare providers to quickly identify the pathogens responsible for the outbreak.Rapid diagnosis allows for early detection and timely implementation of containment measures, such as isolating infected individuals and implementing infection control protocols. During an outbreak, healthcare facilities experience an influx of patients with similar symptoms. Acute care syndromic testing streamlines patient management by providing a comprehensive assessment of the potential pathogens causing the outbreak, allowing for appropriate treatment and care planning. Identifying the causative agent of an outbreak promptly helps limit its spread within the community and healthcare settings. Acute care syndromic testing assists in identifying carriers, isolating cases, and preventing further transmission.
Different infectious agents may require specific treatments. Acute care syndromic testing aids in tailoring treatment plans by providing information about the pathogens involved and their susceptibility to different therapies, which can lead to more effective patient outcomes. Outbreaks often strain healthcare resources. Acute care syndromic testing optimizes resource allocation by quickly identifying cases that require immediate attention and enabling efficient use of medical supplies, personnel, and facilities. Infectious disease outbreaks have highlighted the importance of global health security. Acute care syndromic testing is a critical tool in enhancing preparedness and response capabilities, ensuring a rapid and effective response to emerging threats.
The increasing frequency of global outbreaks such as COVID-19, Zika, Ebola, and emerging influenza strains has underscored the urgent need for robust syndromic testing platforms that can detect a wide array of pathogens within hours. This rapid detection is critical not only for individual patient care but also for public health surveillance, enabling governments and health agencies to monitor the spread of infectious diseases in real time. Acute care syndromic testing systems integrate automation, multiplexing, and high-throughput capabilities, allowing labs and emergency departments to manage high patient volumes while ensuring diagnostic accuracy. These innovations have made syndromic testing essential during crisis scenarios where timely responses can mitigate large-scale outbreaks.
According to the World Health Organization (WHO), infectious diseases cause over 17 million deaths annually, with respiratory infections alone accounting for around 4 million of these cases. This staggering burden emphasizes the need for faster diagnostic interventions in acute settings. The global interconnectedness of travel and trade has increased the speed at which pathogens can cross borders, making syndromic testing a critical first line of defense in hospitals and clinics worldwide. As healthcare systems continue to evolve to handle pandemics and antibiotic resistance, the integration of acute care syndromic testing into emergency protocols is likely to become standard practice, driving sustained growth in the market.
Key Market Challenges
Competition from other diagnostic tests
Syndromic testing competes with other diagnostic tests, such as molecular diagnostics and serological diagnostics. These tests may be more accurate and can provide more information about the underlying cause of the syndrome. molecular diagnostics can identify the specific pathogen causing the syndrome, while serological diagnostics can detect the presence of antibodies to the pathogen. This information can be helpful in making a diagnosis and determining the best course of treatment. Healthcare providers may be more accustomed to using traditional diagnostic methods that they are already familiar with, which can lead to a resistance to adopting newer syndromic testing approaches. Diagnostic methods that have a long history of use and established reliability may be favored by healthcare professionals, making it difficult for syndromic testing to gain traction.Key Market Trends
Rise of Point-of-Care-Testing (POCT)
The rise of Point-of-Care Testing (POCT) is a significant trend in the global Acute Care Syndromic Testing Market. Point-of-Care Testing refers to diagnostic testing performed at or near the patient's location, providing rapid results that can guide immediate clinical decision-making. POCT allows healthcare providers to quickly diagnose and initiate treatment plans, particularly in acute care settings where time is critical. Acute care syndromic testing, when adapted to POCT platforms, enables rapid identification of pathogens responsible for acute symptoms, leading to more timely and appropriate interventions.Syndromic testing conducted at the point of care eliminates the need to send samples to centralized laboratories, reducing turnaround times for results. This speed is essential in managing acute conditions and outbreaks, where rapid decisions are crucial. Point-of-care syndromic testing empowers healthcare providers to make informed decisions at the bedside. Immediate results help guide isolation measures, treatment choices, and patient management strategies, improving overall care. In situations such as infectious disease outbreaks, POCT-based acute care syndromic testing can contribute to rapid containment by quickly identifying cases and implementing appropriate control measures to prevent further spread.
POCT helps optimize resource allocation by avoiding unnecessary transfers, repeat tests, and prolonged hospital stays. This efficiency is especially relevant in acute care scenarios where resources may be limited. During events like large gatherings or disaster response, point-of-care syndromic testing can provide rapid screening for infectious diseases, aiding in quick identification and management of potential outbreaks. POCT can be particularly valuable in remote or underserved regions with limited access to central laboratories. Acute care syndromic testing at the point of care bridges the gap in diagnostic capabilities. Real-time Surveillance: Point-of-care syndromic testing generates data that can be analyzed in real time, contributing to disease surveillance efforts, early detection of emerging pathogens, and timely public health responses.
Key Market Players
- Abbott Laboratories
- Becton, Dickinson and Company
- Biocartis NV
- bioMerieux SA (BioFire Diagnostics)
- Danaher Corporation (Cepheid, Inc.)
- DiaSorin S.p.A (Luminex Corporation)
- Eurofins Scientific (Eurofins Viracor)
- Hologic Inc.
- Laboratory Corporation of America Holdings.
- QIAGEN N.V.
Report Scope:
In this report, the Global Acute Care Syndromic Testing Markets Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Acute Care Syndromic Testing Market, By Disease Type:
- Respiratory Diseases
- Gastrointestinal Diseases
- Genitourinary Diseases
- Tropical Diseases
- Others
Acute Care Syndromic Testing Market, By Target:
- Bacteria
- Fungi
- Viruses
- Parasites
Acute Care Syndromic Testing Market, By Sample Type:
- Blood
- Urine
- Biofluids
- Stool
- Swabs
- Others
Acute Care Syndromic Testing Market, By End User:
- Hospitals
- Clinical & Diagnostics Laboratories
- Research & Academic Institutions
- Others
Acute Care Syndromic Testing Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Major companies present in the Global Acute Care Syndromic Testing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Becton, Dickinson and Company
- Biocartis NV
- bioMerieux SA (BioFire Diagnostics)
- Danaher Corporation (Cepheid, Inc.)
- DiaSorin S.p.A (Luminex Corporation)
- Eurofins Scientific (Eurofins Viracor)
- Hologic Inc.
- Laboratory Corporation of America Holdings.
- QIAGEN N.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | August 2025 |
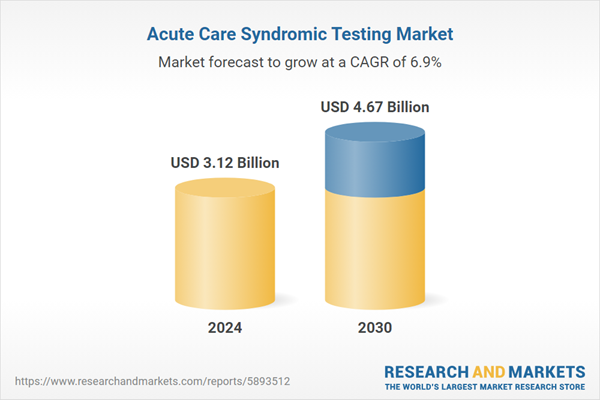
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 3.12 Billion |
| Forecasted Market Value ( USD | $ 4.67 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |