Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
They are utilized to assess the safety of chemicals, drugs, cosmetics, consumer products, and other substances without subjecting animals or humans to potentially harmful effects. These tests provide valuable insights into the potential risks and effects of substances on cellular, molecular, and biochemical levels. In-vitro testing is also often used to screen and prioritize substances for further testing in animal models or clinical trials.
In-vitro toxicology testing has several advantages over traditional animal testing, including ethical considerations, reduced cost and time, and potential for high-throughput screening. However, it also has limitations, such as the inability to fully replicate the complexity of whole organisms and potential differences in responses between in-vitro systems and living organisms. In-vitro Toxicology Testing can be categorized based on cell culture assays, Enzyme Assays, Genotoxicity Assays, Cytotoxicity Assays and High-Throughput Screening (HTS) In-vitro Toxicology Testing.
Key Market Drivers
Rising Demand For Safety Assessment Of New Drugs And Chemicals
The rising demand for safety assessment of new drugs and chemicals is significantly accelerating the adoption of in-vitro toxicology testing across various sectors. According to the U.S. FDA, nearly 70% of investigational new drug (IND) applications rely on non-animal methods, including in-vitro assays, during early screening phases. This underscores a growing trust in laboratory-based models for initial safety profiling. Additionally, a 2023 study published in Nature Reviews Drug Discovery highlighted that over 60% of pharmaceutical companies are now incorporating high-throughput in-vitro assays as part of their standard safety assessment protocols, reflecting a broader industry shift toward more predictive, cost-efficient, and ethically sound testing methodologies.Beyond regulatory mandates, the ability of in-vitro toxicology testing to screen large chemical libraries in parallel using techniques such as high-content imaging and omics technologies has streamlined the early decision-making process in drug development. These tests reduce time-to-market and improve the success rate by identifying cytotoxic, genotoxic, or hepatotoxic risks before clinical trials. Moreover, the integration of human-relevant cell lines and organotypic cultures provides more accurate data on human biological responses, thereby improving the reliability of risk assessments. As precision medicine and chemical safety continue to be prioritized, in-vitro testing is becoming indispensable for safer and more efficient innovation.
The growing complexity and volume of new chemical entities (NCEs) entering research pipelines have also bolstered the importance of in-vitro toxicology testing. As chemical and pharmaceutical industries aim to bring safer products to market faster, in-vitro models help narrow down potential leads by providing critical toxicological profiles early in the development stage. Technologies such as microfluidic "organ-on-chip" platforms are being increasingly integrated to mimic human physiological responses more accurately, allowing researchers to predict organ-specific toxicity with higher precision. This technological advancement has empowered companies to make go/no-go decisions much earlier, saving significant R&D resources and improving product safety outcomes.
Key Market Challenges
Complexity of Biological Systems
The complexity of biological systems poses significant challenges to the global in-vitro toxicity testing market. While in-vitro methods offer numerous advantages, accurately replicating the intricate interactions and dynamic processes that occur within living organisms is a complex endeavor. The challenges arising from biological complexity impact the predictive accuracy, relevance, and applicability of in-vitro toxicity testing.In-vitro models often focus on individual cell types or simplified tissues, which fail to capture the interactions between different organs, tissues, and cell types that occur in the whole organism. This limitation reduces the ability to predict systemic effects and complex physiological responses. Cells in the body interact within a specific microenvironment, including extracellular matrix, signaling molecules, and neighboring cells. Replicating these interactions in in-vitro models is challenging, potentially leading to altered cellular behavior and responses.
Additionally, the metabolic capacity of in-vitro systems often falls short compared to that of an entire organism. Many toxic effects arise from metabolites generated during the body's metabolic processes, particularly in the liver. Standard in-vitro models may not accurately reproduce these metabolic transformations, leading to an underestimation or misinterpretation of a substance’s toxicity. For instance, hepatocyte cultures may not fully reflect the enzymatic activity of a functioning liver, which is crucial for assessing the safety of drugs and chemicals.
Another layer of complexity is introduced by individual genetic variability. Humans exhibit differences in gene expression, metabolism, and immune responses, all of which influence how substances are processed in the body. Most in-vitro systems use standardized cell lines that do not capture this inter-individual variability. This presents a limitation in predicting population-wide safety outcomes and personalizing risk assessments. As a result, despite advances in 3D cultures and organ-on-chip technologies, translating in-vitro findings to real-world human scenarios remains a significant hurdle for researchers and regulatory bodies alike.
Key Market Trends
Personalized Medicine Applications
Personalized medicine applications represent a significant trend in the global in-vitro toxicity testing market. Personalized medicine aims to tailor medical treatment to the individual characteristics of each patient, including their genetic makeup, lifestyle, and environmental factors. In the context of in-vitro toxicity testing, personalized medicine applications involve assessing how an individual's unique genetic and physiological characteristics influence their response to potential toxicants. In-vitro toxicity testing can be used to evaluate how a patient's specific genetic and molecular profile influences their susceptibility to adverse effects from chemicals and drugs.This approach enables more accurate and personalized risk assessments, helping to identify individuals who may be particularly sensitive to certain substances. By using patient-derived cells or tissues, researchers can conduct in-vitro toxicity testing to predict how an individual's body might respond to a particular compound. This information can guide treatment decisions and drug choices to maximize efficacy and minimize risks for each patient.
In-vitro toxicity testing can help identify biomarkers or specific molecular indicators that signal potential toxic responses in certain individuals. These biomarkers can be used to monitor and predict toxicity in real-time during treatment. In-vitro toxicity testing can play a crucial role in identifying compounds that may lead to adverse reactions in specific patient populations. By selecting safer alternatives based on personalized testing, the risk of adverse effects can be significantly reduced.
Key Market Players
- Charles River Laboratories International, Inc.
- SGS S.A.
- Merck KGaA
- Eurofins Scientific
- Abbott Laboratories
- Laboratory Corporation of America Holdings
- Evotec S.E.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies, Inc.
Report Scope:
In this report, the Global In-vitro Toxicology Testing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:In-vitro Toxicology Testing Market, By Technology:
- Cell Culture Technology
- High Throughput Technology
- Molecular Imaging
- OMICS Technology
In-vitro Toxicology Testing Market, By Application:
- Systemic Toxicology
- Dermal Toxicity
- Endocrine Disruption
- Occular Toxicity
- Others
In-vitro Toxicology Testing Market, By Method:
- Cellular Assay
- Biochemical Assay
- In-silico
- Ex-vivo
In-vitro Toxicology Testing Market, By End User:
- Pharmaceutical Industry
- Cosmetics & Household Products
- Academic Institutes & Research Laboratories
- Diagnostics
- Chemicals Industry
- Food Industry
In-vitro Toxicology Testing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global In-vitro Toxicology Testing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Charles River Laboratories International, Inc.
- SGS S.A.
- Merck KGaA
- Eurofins Scientific
- Abbott Laboratories
- Laboratory Corporation of America Holdings
- Evotec S.E.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | August 2025 |
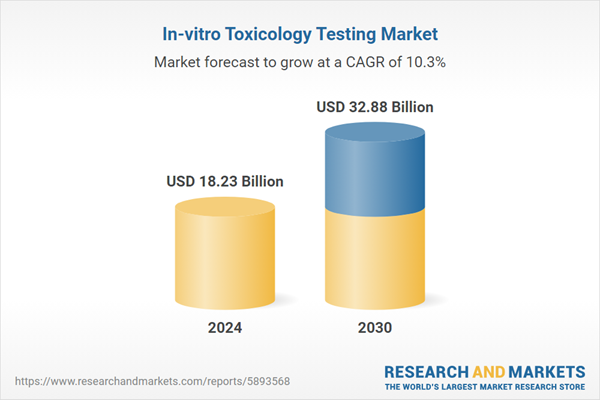
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 18.23 Billion |
| Forecasted Market Value ( USD | $ 32.88 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |