Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Increasing prevalence of chronic diseases
The increasing prevalence of chronic diseases is one of the key factors driving the growth of the Canada clinical biomarkers market. Chronic diseases, such as cancer, diabetes, and cardiovascular diseases, are a major public health concern in Canada, and they are responsible for a significant burden on the healthcare system. Clinical biomarkers play a critical role in the early diagnosis, monitoring, and treatment of chronic diseases. Biomarkers are measurable indicators of disease, and they can help to identify individuals who are at high risk of developing a disease or who have already developed a disease. Biomarkers can also be used to monitor disease progression, predict treatment response, and assess the efficacy of a therapy. The increasing prevalence of chronic diseases is driving the demand for clinical biomarkers in Canada. Biomarkers are becoming an essential tool for disease diagnosis and management, and their adoption is expected to increase in the coming years. The use of biomarkers can help to improve patient outcomes, reduce healthcare costs, and increase the efficiency of the healthcare system. The growing demand for personalized medicine is also contributing to the growth of the Canada clinical biomarkers market. Personalized medicine involves the use of biomarkers to identify patients who are likely to respond to specific therapies. Biomarkers can help to predict treatment response, monitor treatment efficacy, and select the most appropriate therapy for a patient. Personalized medicine is likely to become more prevalent in the future, which is expected to drive the demand for clinical biomarkers during the forecast period.Growing demand for personalized medicine
The growing demand for personalized medicine is a significant driver of the Canada clinical biomarkers market. Personalized medicine involves the use of biomarkers to identify patients who are likely to respond to specific therapies, making the treatment more effective and efficient. Biomarkers are measurable indicators of disease or physiological processes, and they can help to predict treatment response, monitor treatment efficacy, and select the most appropriate therapy for a patient. The use of clinical biomarkers is critical to the success of personalized medicine. Biomarkers can help to identify patients who are most likely to respond to a specific therapy, reducing the need for trial and error in the treatment selection process. Biomarkers can also help to monitor the response to therapy, allowing clinicians to make adjustments as needed to optimize treatment outcomes. The growing demand for personalized medicine is expected to drive the adoption of clinical biomarkers in Canada during the forecast period. The healthcare industry is shifting toward a more personalized approach to treatment, and the use of biomarkers is expected to become increasingly prevalent in the coming years. The development of new biomarkers and the improvement of existing ones will be critical to the success of personalized medicine, and the Canada clinical biomarkers market is poised to play a key role in this shift. The adoption of clinical biomarkers in personalized medicine has the potential to improve patient outcomes, reduce healthcare costs, and increase the efficiency of the healthcare system. Clinical biomarkers can help to identify patients who are most likely to respond to a specific therapy, reducing the need for expensive and potentially ineffective treatments. The use of biomarkers can also help to identify patients who are at high risk of developing a disease, allowing for early intervention and improved outcomes.Technological advancements
Technological advancements are driving the growth of the Canada clinical biomarkers market. Advances in technology have led to the development of new biomarkers, as well as improvements in the sensitivity and specificity of existing biomarkers. These technological advancements have expanded the range of biomarkers that can be used for clinical diagnosis and treatment monitoring and have improved the accuracy and reliability of biomarker-based tests. One key technological advancement in the field of clinical biomarkers is the use of high-throughput screening technologies. These technologies allow for the rapid and simultaneous analysis of large numbers of biomarkers, enabling clinicians to identify biomarker profiles that are indicative of specific diseases or conditions. High-throughput screening technologies have greatly expanded the range of biomarkers that can be used for clinical diagnosis and treatment monitoring and have facilitated the development of personalized medicine approaches. Another important technological advancement in the field of clinical biomarkers is the development of multiplexed assay technologies. These technologies enable the simultaneous analysis of multiple biomarkers in a single sample, providing a more comprehensive profile of a patient's disease status. Multiplexed assay technologies have improved the accuracy and efficiency of biomarker-based tests and have facilitated the development of personalized medicine approaches. In addition to these advancements in technology, the growing availability of big data analytics tools and artificial intelligence (AI) is also driving the growth of the Canada clinical biomarkers market. These tools enable the analysis of large amounts of data to identify biomarker profiles and to develop predictive models for disease diagnosis and treatment monitoring. The use of big data analytics and AI is expected to revolutionize the field of clinical biomarkers, enabling more accurate and efficient disease diagnosis and treatment monitoring.Increasing healthcare expenditure
The increasing healthcare expenditure is a significant driver for the growth of the Canada clinical biomarkers market. Healthcare expenditure refers to the total amount of money spent on healthcare services, including diagnosis, treatment, and prevention of diseases. As healthcare expenditure increases, there is a corresponding increase in the demand for advanced diagnostic tools and personalized treatment options, which includes the use of clinical biomarkers. Clinical biomarkers play an essential role in the diagnosis, treatment, and monitoring of various diseases. They provide important information about a patient's disease status, response to treatment, and potential side effects. As the demand for advanced diagnostic tools and personalized treatment options continues to increase, the use of clinical biomarkers is expected to grow in parallel. The increasing healthcare expenditure is also driving the development and adoption of new biomarkers, which is further contributing to the growth of the Canada clinical biomarkers market. The development of new biomarkers requires significant investment in research and development, which is often funded through healthcare expenditure. With increased funding, researchers are able to develop new biomarkers that can be used for early disease detection, personalized treatment, and improved patient outcomes. Furthermore, the increasing healthcare expenditure is leading to the adoption of advanced healthcare technologies, including clinical biomarkers. Healthcare providers are investing in new diagnostic tools and treatment options that are more efficient, accurate, and cost-effective. Clinical biomarkers offer a highly targeted and personalized approach to diagnosis and treatment, which aligns with the goals of the healthcare industry to provide high-quality and cost-effective care to patients.Government initiatives
Government initiatives play a significant role in driving the growth of the Canada clinical biomarkers market. The Canadian government has implemented several initiatives to promote the development and use of clinical biomarkers in healthcare. One such initiative is the Strategic Innovation Fund, which provides funding to support research and development activities in a variety of sectors, including healthcare. This funding can be used to support the development of new biomarkers, as well as the validation and commercialization of existing biomarkers. The Strategic Innovation Fund has helped to spur innovation in the clinical biomarkers industry, leading to the development of new and improved biomarkers that can be used for diagnosis and treatment monitoring. Another government initiative that is driving the growth of the Canada clinical biomarkers market is the Canadian Institutes of Health Research (CIHR). The CIHR provides funding for research in various areas of health, including the development of new biomarkers. This funding has helped to support the development of new biomarkers that can be used for diagnosis and treatment monitoring and has led to the advancement of personalized medicine approaches. The Canadian government has also implemented regulations and guidelines to ensure the safety and effectiveness of clinical biomarkers. For example, Health Canada regulates the approval and use of medical devices, including biomarkers, to ensure their safety and efficacy. These regulations and guidelines help to promote the development of high-quality biomarkers that can be used safely and effectively in healthcare. Furthermore, the Canadian government has implemented initiatives to improve access to healthcare services, including the use of clinical biomarkers. For example, the Canada Health Infoway initiative has been implemented to promote the adoption of digital health technologies, including the use of biomarkers, to improve healthcare delivery and patient outcomes.Market Segmentation
The Canada clinical biomarkers market can be segmented by product, services, technology, clinical area, application, end user, and region. Based on product, the Canada clinical biomarkers market can be segmented into efficacy biomarkers, safety biomarkers, and validation biomarkers. Based on services, the Canada clinical biomarkers market can be segmented into genomic biomarker service, tissue biomarker service, cell service, and proteomics service. Based on technology, the Canada clinical biomarkers market can be segmented into next generation sequencing, polymerase chain reaction, immunohistochemistry, enzyme-linked immunosorbent assay, and others. Based on clinical area, the Canada clinical biomarkers market can be segmented into cancer biomarker, cardiac biomarker, neurological biomarker, infectious disease biomarker, and others. Based on application, the Canada clinical biomarkers market can be divided into translational research v/s clinical diagnostics. Based on end user, the Canada clinical biomarkers market can be segmented into biotechnology & pharmaceutical companies, diagnostic centers, academic & research institutions, and others.Market Players
Agilent Technologies Canada, Hoffmann-La Roche Limited., QIAGEN NV, PerkinElmer Canada Inc, Thermo Fisher Scientific Inc., Bio-Rad Laboratories Canada Ltd., and Abbott Medical Canada Inc. are some of the leading players operating in the Canada clinical biomarkers market.Report Scope:
In this report, the Canada clinical biomarkers market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Canada Clinical Biomarkers Market, By Product:
- Efficacy Biomarkers
- Safety Biomarkers
- Validation Biomarkers
Canada Clinical Biomarkers Market, By Services:
- Genomic Biomarker Service
- Tissue Biomarker Service
- Cell Service
- Proteomics Service
Canada Clinical Biomarkers Market, By Technology:
- Next Generation Sequencing
- Polymerase Chain Reaction
- Immunohistochemistry
- Enzyme-Linked Immunosorbent Assay
- Others
Canada Clinical Biomarkers Market, By Clinical Area:
- Cancer Biomarker
- Cardiac Biomarker
- Neurological Biomarker
- Infectious Disease Biomarker
- Others
Canada Clinical Biomarkers Market, By Application:
- Translational Research
- Clinical Diagnostics
Canada Clinical Biomarkers Market, By End User:
- Biotechnology & Pharmaceutical Companies
- Diagnostic Centers
- Academic & Research Institutions
- Others
Canada Clinical Biomarkers Market, By Region:
- Ontario region
- Quebec region
- Alberta region
- British Columbia region
- Saskatchewan and Manitoba region
- Rest of Canada
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present Canada clinical biomarkers market.Available Customizations:
With the given market data, the publisher offers customizations according to a company’s specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Agilent Technologies Canada
- Hoffmann-La Roche Limited.
- QIAGEN NV
- PerkinElmer Canada Inc
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories Canada Ltd.
- Abbott Medical Canada Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 89 |
| Published | October 2023 |
| Forecast Period | 2023 - 2028 |
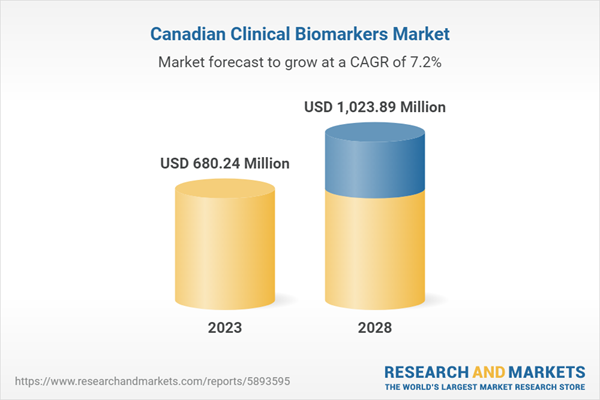
| Estimated Market Value ( USD | $ 680.24 Million |
| Forecasted Market Value ( USD | $ 1023.89 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Canada |
| No. of Companies Mentioned | 7 |