Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive and practical orientation to how artificial intelligence integrates into pathology workflows, clinical decision-making, and organizational strategy
Artificial intelligence is reshaping pathology through advances in image analysis, predictive modeling, and workflow optimization, establishing new pathways for clinical insight and operational efficiency. This introduction presents the technological foundations, clinical reasoning, and organizational imperatives that underpin AI adoption in pathology, emphasizing how algorithmic tools intersect with human expertise to enhance diagnostic accuracy and throughput. By focusing on clinically meaningful endpoints, such as diagnostic concordance, turnaround time, and reproducibility, stakeholders can align technical capabilities with clinician needs and patient outcomes.Moreover, the introduction outlines the ecosystem of stakeholders that must coordinate to realize value: pathology laboratories, hospital leadership, medical device manufacturers, software developers, regulatory bodies, and academic centers. It highlights the importance of robust data pipelines, validated imaging modalities, and interoperable IT architectures that enable secure data exchange and reproducible results. Finally, it frames the subsequent sections of the report by identifying key topics for deeper exploration - technology shifts, policy and tariff impacts, segmentation insights, regional variations, competitive dynamics, and actionable recommendations - thereby preparing readers to navigate both immediate decisions and longer-term strategic planning.
An in-depth analysis of converging technological, clinical, and commercial forces that are redefining pathology practice and enabling scalable AI-driven capabilities
The landscape of pathology is undergoing transformative shifts driven by improvements in digital imaging, machine learning methodologies, and the maturation of clinical validation pathways. Key technological inflection points include the emergence of high-resolution whole slide imaging that preserves diagnostic detail, the proliferation of specialized data analysis software optimized for histopathologic patterns, and the refinement of workflow management tools that prioritize case triage and resource allocation. Together, these capabilities enable a transition from analog slide review toward integrated computational pathology solutions that augment human expertise.Concurrently, clinical practice is evolving as digital pathology and telepathology expand the geographic reach of subspecialty consultations and support more flexible service models. Predictive analytics are being embedded into diagnostic pathways to support prognostic models and risk prediction that inform therapeutic decision-making. At the same time, industry dynamics are shifting: strategic partnerships between hardware manufacturers and software developers are becoming more common, while professional services and training offerings mature to help laboratories scale deployments. These converging trends create a powerful impetus for laboratories and health systems to re-evaluate their diagnostic architectures and to invest in interoperable platforms that can support evolving clinical and operational requirements.
A strategic assessment of how recent tariffs reshaped procurement, supply chains, and deployment choices for imaging hardware and associated AI solutions throughout 2025
Recent tariff changes implemented in 2025 have had a cumulative effect on the supply chains and capital planning decisions for organizations deploying advanced pathology equipment and services. Tariffs that increase the landed cost of hardware components such as high-resolution scanners, servers, and specialized imaging devices introduce measurable pressure on procurement budgets and can extend vendor qualification timelines. As a result, laboratories and hospital networks face trade-offs between accelerating digitization programs and preserving capital for other clinical priorities.In response, procurement teams are adapting by re-evaluating total cost of ownership, negotiating bundled offerings that include hardware, software, and professional services, and considering alternative deployment models that shift capital expenses to operational expense structures. Cloud-based deployment options, where regulatory and data governance constraints permit, are attracting interest because they can reduce upfront hardware acquisition needs. Additionally, some vendors are adjusting supply chain strategies, diversifying manufacturing footprints, and revising pricing models to absorb portions of the tariff impact. These adaptations collectively influence the pace and shape of AI adoption in pathology, prompting more nuanced capital planning and partnership choices across clinical and commercial organizations.
A detailed segmentation-driven perspective that maps products, applications, end users, and deployment modes to practical adoption pathways and development priorities
A granular segmentation analysis clarifies where clinical need, technological capability, and commercial models intersect, offering practical guidance for prioritization and product development. Based on product type, the landscape divides into service-oriented and solution-oriented offerings. Services encompass professional services that support system integration and validation as well as training and support programs that build operational competency. Solutions encompass both hardware and software components; hardware includes scanning and imaging devices plus compute infrastructure, while software spans data analysis applications, whole slide imaging systems, and workflow management platforms designed to orchestrate case assignment and reporting.From an application standpoint, the technology portfolio is applied across computational pathology, which focuses on algorithmic interpretation of image and molecular data; digital pathology, which includes telepathology and whole slide imaging to enable remote review and collaboration; predictive analytics, which supports prognostic models and patient-specific risk prediction; and workflow optimization, which targets case triage and resource allocation to improve throughput. End users reflect this diversity: diagnostic laboratories serve both hospital-based and reference laboratory models, hospitals and clinics include both large tertiary centers and small to mid-size facilities, pharmaceutical and biotechnology organizations range from startups to large pharma engaged in drug development and companion diagnostics, and research institutes include academic centers and private labs that pursue translational research.
Deployment mode is an additional axis that informs procurement and implementation strategies, distinguishing between cloud and on-premise solutions. Cloud deployments can utilize private cloud configurations where data segmentation and control are paramount or public cloud services that emphasize elasticity and rapid scalability. Conversely, on-premise deployments remain attractive for institutions with strict data residency or regulatory constraints, requiring carefully architected integration with laboratory information systems and digital slide archives. By synthesizing these segmentation layers, stakeholders can identify specific product and service configurations that align with clinical workflows, regulatory contexts, and organizational resources.
A comparative regional analysis highlighting how clinical priorities, regulatory frameworks, and deployment preferences shape adoption trajectories across global territories
Regional dynamics materially influence how AI in pathology is adopted, funded, and regulated, creating differentiated opportunities and barriers across global health systems. In the Americas, there is a strong emphasis on clinical validation, reimbursement pathways, and private-sector partnerships that support commercial deployments in both hospital systems and reference laboratory networks. Regulatory engagement in this region tends to be rigorous, necessitating high levels of evidence and well-documented performance characteristics to support clinical integration.Across Europe, the Middle East & Africa, the landscape is heterogeneous: certain markets emphasize centralized digital pathology networks and cross-border telepathology services, while others are focused on capacity-building and workforce training. Data protection frameworks and regional regulatory harmonization initiatives are shaping how cloud and cross-border deployments are structured, making compliance and localized partnerships critical considerations. In the Asia-Pacific region, rapid digitization in large tertiary centers coexists with growing investment in regional reference facilities and research institutes. Stakeholders in this region often prioritize scalable, cost-effective solutions and are receptive to public-private collaborations that accelerate clinical validation and adoption. Understanding these regional priorities enables vendors and clinical leaders to tailor value propositions, deployment models, and stakeholder engagement strategies to local contexts and regulatory expectations.
A nuanced competitive overview that explains how device manufacturers, software innovators, startups, and clinical collaborators are shaping the AI pathology ecosystem
Competitive dynamics in AI-enabled pathology reflect a mixed ecosystem of established device manufacturers, specialized software vendors, agile startups, and cross-disciplinary partnerships with clinical and research institutions. Hardware incumbents continue to leverage their distribution networks and clinical relationships to bundle imaging systems with analytics, while software-focused companies differentiate through advanced algorithms, user experience design, and interoperability capabilities that integrate with laboratory information systems and electronic health records.Startups often lead in niche innovation areas such as algorithmic subtyping, prognostic modeling, and novel annotation tools, and they frequently partner with academic centers to generate clinical evidence. Pharmaceutical and biotechnology firms act as important collaborators, contributing annotated datasets and participating in co-development projects for companion diagnostics. Professional service firms and system integrators play a pivotal role in validation, change management, and training, enabling laboratories to translate pilot results into scaled operations. Collectively, these players form a dynamic value chain in which strategic alliances, regulatory acumen, and demonstrated clinical impact are critical differentiators for long-term success.
Clear and actionable strategic recommendations for healthcare executives, laboratory leaders, and technology providers to accelerate adoption and ensure sustainable integration of AI
Industry leaders should pursue a set of pragmatic actions to accelerate adoption while mitigating operational and regulatory risks. First, prioritize clinical validation strategies that produce reproducible, peer-reviewed evidence on diagnostic performance and clinical utility; invest in multi-institutional studies and prospective workflows that reflect real-world practice. Second, adopt modular integration approaches that emphasize interoperability with laboratory information systems and electronic health records to reduce friction and protect existing investments. Third, explore flexible commercial models that align vendor incentives with institutional outcomes, including subscription arrangements, bundled services, and outcome-linked agreements where feasible.Additionally, develop a robust data governance framework that addresses privacy, provenance, and model maintenance, and invest in workforce development programs to ensure pathologists and laboratory staff have practical training in digital workflows and AI interpretation. Engage early with regulatory bodies to clarify requirements for clinical deployment and to streamline approval pathways. Finally, cultivate strategic partnerships across academia, industry, and clinical networks to access high-quality data, accelerate algorithm refinement, and distribute implementation risk. By following these recommendations, organizations can build resilient strategies that balance innovation with operational sustainability.
A transparent and rigorous research methodology that integrates literature review, stakeholder interviews, technology assessment, and expert validation to ensure actionable insights
The research methodology underpinning this report combined multiple qualitative and quantitative techniques to ensure a balanced and evidence-based analysis. The approach began with a comprehensive review of peer-reviewed literature, regulatory guidance, technical standards, and clinical practice guidelines to establish a foundation of validated knowledge. This was complemented by structured interviews and workshops with a diverse set of stakeholders, including practicing pathologists, laboratory directors, clinical informaticists, device manufacturers, software developers, and regulatory specialists, to capture real-world perspectives and operational constraints.Technology assessment methods were used to evaluate imaging hardware characteristics, algorithmic performance metrics, software interoperability, and deployment models, while case studies illuminated implementation pathways and lessons learned from early adopters. Data synthesis involved triangulating findings across primary and secondary sources to identify convergent themes, and validation sessions with domain experts were conducted to refine interpretations and recommendations. Throughout, emphasis was placed on transparency of assumptions, reproducibility of analytical steps, and alignment with clinical practice realities to ensure the research outputs are both rigorous and actionable for decision-makers.
A concise and forward-looking conclusion that synthesizes clinical, technological, and operational imperatives for unlocking the value of AI in pathology
In summary, artificial intelligence in pathology represents a pivotal inflection point for diagnostic medicine, offering demonstrable potential to enhance accuracy, accelerate workflows, and support personalized care pathways. The convergence of high-resolution imaging, specialized data analysis software, and workflow management tools is enabling new clinical capabilities, while evolving regulatory and reimbursement contexts are shaping the path to routine adoption. Organizations that proactively align validation strategies, data governance, workforce development, and interoperable technology architectures will be positioned to realize the greatest clinical and operational benefits.Looking ahead, progress will depend on collaborative models that combine clinical rigor with technical innovation, transparent evidence generation, and attentive change management. By understanding segmentation nuances, regional priorities, competitive dynamics, and the implications of policy shifts such as tariffs, stakeholders can make informed choices that balance risk and opportunity. Ultimately, the disciplined application of AI in pathology has the potential to improve patient outcomes and create more resilient diagnostic ecosystems when implemented with thoughtful governance and clinical oversight.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Artificial Intelligence in Pathology Market
Companies Mentioned
The key companies profiled in this Artificial Intelligence in Pathology market report include:- aetherAI
- Aiforia Technologies Oyj
- Akoya Biosciences, Inc.
- Danaher Corporation
- Deep Bio, Inc.
- Evident Corporation
- F. Hoffmann-La Roche Ltd.
- Ibex Medical Analytics Ltd.
- Indica Labs, Inc.
- Inspirata, Inc.
- Koninklijke Philips N.V.
- LUMEA, Inc.
- MindPeak GmbH
- Nucleai Inc.
- OptraSCAN Inc.
- Paige.AI, Inc.
- PathAI, Inc.
- Proscia Inc.
- Siemens Healthineers AG
- Techcyte, Inc.
- Tempus Labs, Inc.
- Tribun Health
- Visikol, Inc. by CELLINK
- Visiopharm A/S
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
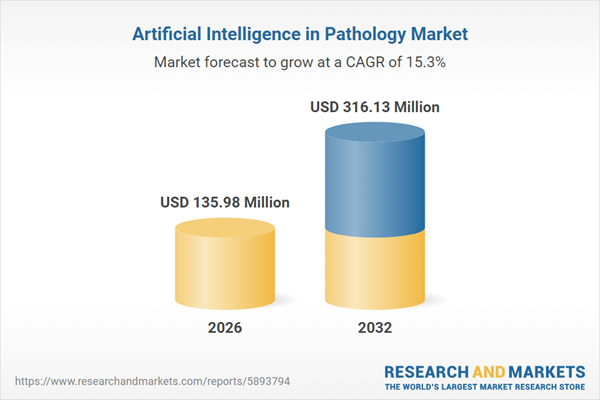
| Estimated Market Value ( USD | $ 135.98 Million |
| Forecasted Market Value ( USD | $ 316.13 Million |
| Compound Annual Growth Rate | 15.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |