Tricuspid Valve Repair: Introduction
Surgeries that are performed to treat damaged or diseased tricuspid valve are known as tricuspid valve repair. It is one of four valves that control blood flow through the heart. It separates the upper and lower right chambers of the heart (right atrium and right ventricle). A damaged or diseased tricuspid valve can interfere with the proper direction of blood flow. The heart must work harder to send blood to the lungs and the rest of the body.Tricuspid valve repair and tricuspid valve replacement can help improve blood flow and reduce symptoms of heart valve disease. Tricuspid valve repair or replacement may be done as open-heart surgery or as a minimally invasive heart surgery. Sometimes, tricuspid valve disease may be treated with a catheter-based procedure.
Global Tricuspid Valve Repair Market Analysis
The increasing technological advancements in the treatment approaches for tricuspid valve repair is expected to influence the market growth. The pharmaceutical companies are increasingly participating in the development of new technologies and products that are expected to boost the global tricuspid valve repair market growth. For instance, Abbott has released TriClip™ transcatheter edge-to-edge repair (TEER) system, which is a first-of-its kind minimally invasive device, designed specifically for tricuspid heart valve repair. After getting through several observations, this new product has proven it efficacy with high percentage.The increase in per-person healthcare spending across the globe is anticipated to expand the market growth. The expanding utilization of annuloplasty systems that are implanted around an incompetent cardiac valve to revive the blood flow in heart is expected to directly contribute to the market expansion.
Global Tricuspid Valve Repair Market Segmentations
Tricuspid Valve Repair Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Procedure Type
- Surgical Repair
- Transcatheter Repair
Market Breakup by Patient Condition
- Valvular Disease
- Structural Abnormalities
Market Breakup by Approach
- Traditional
- Minimally Invasive
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centers
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Tricuspid Valve Repair Market Overview
The increasing prevalence of cardiovascular diseases globally is driving the market growth as they are the leading cause of cardiovascular morbidity and mortality and a major factor responsible for symptoms and functional disability. The adoption of minimally invasive surgical procedures is also driving the market growth. The increasing integration of technological advancement in the surgical equipment for tricuspid are driving the growth of the global tricuspid valve repair market. The integration of advanced technologies such as artificial intelligence AI is expected to boost the global tricuspid valve repair market growth as it helps to enhance the surgical decision-making before, after, and even during a surgical procedure. The increasing geriatric population is expected to boost the market growth as well. The research and development activities are also increasing globally to find better and effective solutions for tricuspid valve repair surgery, further propelling the market growth.Geographically, North America is leading the regional market for tricuspid valve repair. The increasing prevalence of cardiovascular diseases is contributing to the market growth. Nearly 16.3 million Americans aged 20 and older suffer with Congenital Heart Defects CHDs with a 7% prevalence rate. The prevalence for men and women is 8.3% and 6.1% respectively, propelling the market growth.
Tricuspid Valve Repair Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Edwards Lifesciences Corporation
- Medtronic plc.
- Abbott Laboratories
- Boston Scientific Corporation
- LivaNova PLC
- CryoLife Inc.
- Micro Interventional Devices, Inc.
- Neovasc Inc.
- NaviGate Cardiac Structures Inc.
- TTK Healthcare Limited
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Edwards Lifesciences Corporation
- Medtronic plc.
- Abbott Laboratories
- Boston Scientific Corporation
- LivaNova PLC
- CryoLife Inc.
- Micro Interventional Devices, Inc.
- Neovasc Inc.
- NaviGate Cardiac Structures Inc.
- TTK Healthcare Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
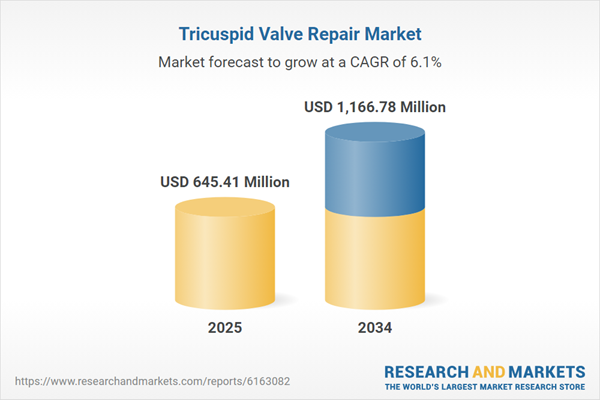
| Estimated Market Value ( USD | $ 645.41 Million |
| Forecasted Market Value ( USD | $ 1166.78 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |