Castrate-Resistant Prostate Cancer: Introduction
Castrate-Resistant Prostate Cancer (CRPC) is an advanced type of prostate cancer wherein the body stops responding to treatments. Prostate cancer is commonly treated by blocking the male sex hormone, testosterone, that promotes cancer cells growth. However, in the case of advanced castrate-resistant prostate cancer, the body stops reacting to hormone therapy.CRPC can be metastatic or nonmetastatic. In metastatic CRPC (mCRPC), cancer spreads to other parts of the body. Common symptoms include pain while urinating, blood in urine, weight loss, fatigue, or shortness of breath. The treatment involves slowing the cancer growth and controlling the symptoms with the help of chemotherapy, immunotherapy, gene therapy and others.
In non-metastatic CRPC (nmCRPC), the cancer is only present in the prostate region.
Castrate-Resistant Prostate Cancer Market Analysis
Prostate cancer is the second most prominent cancer type in males affecting approximately 15.1% of the population. The treatment consists of radiation, chemotherapy, hormone therapy, targeted therapy, immunotherapy, and gene therapy. Hormone therapy is mostly preferred and constitutes treatments like androgen deprivation therapy (ADT), bilateral orchiectomy, LHRH agonists, androgen receptor (AR) inhibiting drugs such as Apalutamide, Darolutamide and Enzalutamide, and GnRH antagonists. Although these treatments have brought major improvements, there are several side effects one may experience with it. Hence, there has been extensive research in the domain fuelling castrate-resistant prostate cancer market value.Radioligand therapy has gained attention of the scientists in the recent years. It sends radioactive emitters directly to the tumour associated targets. These radioactive atoms only target the DNA of cancer cells and provides an edge over the conventional external-beam radiation therapy, which cannot target the tumour cells directly. Currently, a combination of 177Lu-PSMA-617 with actinium-225 in conjunction to the J591 monoclonal antibody, is under first and second phase trials.
Molecular targeted therapy is another latest and advancing fields of CRPC treatment. In August 2024, FDA approved the combination of Enzalutamide (Xtandi) with Talazoparib (Talzenna) to treat metastatic Castrate-Resistant Prostate Cancer. Enzalutamide works by blocking hormones that accelerate cancer cell growth followed by Talazoparib, which blocks the activities of PARP (DNA repairing) protein. This makes cancer cell survival difficult. Talazoparib is the third PARP blocking drug to receive FDA approval. As a result, the castrate-resistant prostate cancer market growth is certain with the increasing research and development in oncology.
Castrate-Resistant Prostate Cancer Market Segmentation
“Castrate-Resistant Prostate Cancer Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Therapy Type
- Hormonal Therapy
- Immunotherapy
- Chemotherapy
- Others
Market Breakup by Therapy Drug Class
- Antineoplastic
- Non-steroidal Antiandrogen
- Corticosteroids
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Market Breakup by Region- 7MM
- United States
- EU-4 and the United Kingdom
- Japan
Castrate-Resistant Prostate Cancer Market Overview
The North American territory dominates the castrate-resistant prostate cancer market share as it is the second leading cause of cancer death in the region. With the prevalence of a geriatric population and unhealthy lifestyle changes, the number of morbidities has grown in the area. Therefore, the government and key players of healthcare industry have taken strict measures to spread awareness about the disease in the relevant population. The presence of a proper infrastructure also enables the scientists to continue research for effective treatment.The European territory along with Asia-pacific region can also observe a significant surge in the market, especially due to the efforts of the government and educational institutes to boost effective research and development. The Asian region being home world's largest geriatric population can be a large market in the forecast period.
Castrate-Resistant Prostate Cancer Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Sanofi
- Johnson & Johnson Services Inc.
- Pfizer Inc.
- Astellas Pharma, Inc.
- Bayer AG
- F. Hoffmann-La Roche Ltd.
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- GlaxoSmithKline plc
- Novartis AG
- Eli Lilly and Company
- Merck & Co., Inc.
- Allergan
- AstraZeneca
- Cipla Inc.
- Amneal Pharmaceuticals LLC
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Sanofi
- Johnson & Johnson Services Inc.
- Pfizer Inc.
- Astellas Pharma, Inc.
- Bayer AG
- F. Hoffmann-La Roche Ltd.
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- GlaxoSmithKline plc
- Novartis AG
- Eli Lilly and Company
- Merck & Co., Inc.
- Allergan
- AstraZeneca
- Cipla Inc.
- Amneal Pharmaceuticals LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
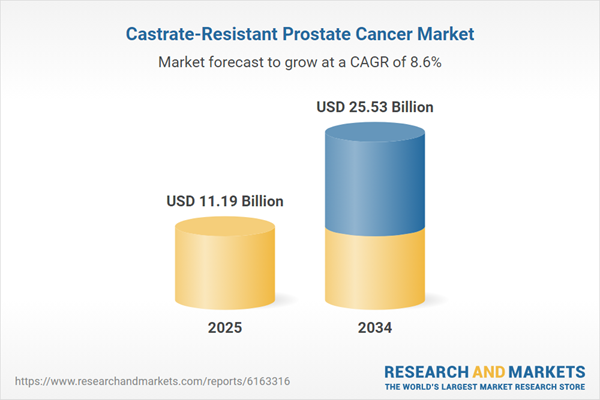
| Estimated Market Value ( USD | $ 11.19 Billion |
| Forecasted Market Value ( USD | $ 25.53 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |