Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
As advancements in stem cell research continue, their clinical applications are expanding rapidly, particularly in hematopoietic transplants and regenerative therapies. Rising demand for cell-based treatments, alongside increased investments and collaborations in R&D, is accelerating the development of scalable and standardized manufacturing technologies. Moreover, regulatory support and innovations in cell banking and bioprocessing are enhancing market growth. Despite challenges like regulatory complexity and high production costs, the global market is progressing with the emergence of automated systems, contract manufacturing, and high-throughput technologies.
Key Market Drivers
Increasing Prevalence of Chronic Diseases
The growing incidence of chronic conditions such as diabetes, cardiovascular disorders, neurodegenerative diseases, and autoimmune illnesses is significantly driving demand for stem cell therapies. These disorders often result in irreversible tissue or organ damage, where conventional treatments fall short. Stem cell-based therapies offer regenerative capabilities that can repair or replace damaged tissues, making them a promising option for patients with limited therapeutic alternatives. With a rising patient population and associated healthcare burden, especially in aging societies, there is a heightened focus on innovative regenerative solutions. The expansion of clinical trials and supportive regulatory frameworks aimed at facilitating access to stem cell treatments is further bolstering the demand for advanced manufacturing processes capable of delivering safe, scalable, and high-quality therapeutic cells.Key Market Challenges
Regulatory Complexities
Navigating the regulatory landscape for stem cell manufacturing remains a significant hurdle. Differing global regulations and the need for rigorous safety and efficacy evaluations slow down the development and commercialization of stem cell therapies. Regulatory bodies require extensive clinical and preclinical data, along with ongoing safety monitoring, which can delay product approvals and increase operational costs. Moreover, the ethical concerns associated with certain stem cell sources, like embryonic stem cells, further complicate the process, particularly in regions with restrictive policies. Compliance with Good Manufacturing Practices (GMP), implementing quality control systems, and managing global variability in legal frameworks can be resource-intensive, especially for emerging players, limiting their market entry and scalability.Key Market Trends
Advanced Bioprocessing Technologies
The stem cell manufacturing industry is witnessing a significant shift towards advanced bioprocessing technologies that support scalability, cost reduction, and quality assurance. Automated bioreactor systems, closed-loop platforms, and real-time monitoring solutions are transforming stem cell production by enhancing consistency and minimizing contamination risks.These technologies allow for the high-throughput generation of therapeutic-grade cells while ensuring regulatory compliance and product standardization. Innovations in upstream and downstream processing, such as improved cryopreservation, cell expansion methods, and integrated monitoring tools, are further enabling faster production cycles and improved safety profiles. As demand for stem cell therapies increases, especially in personalized and allogeneic applications, advanced bioprocessing systems are becoming central to commercial viability and broader market adoption.
Key Market Players
- Thermo Fisher Scientific.
- Merck KGaA.
- AbbVie Inc.
- CO. LTD.
- Astellas Pharma Inc.
- Bristol-Myers Squibb Company.
- FUJIFILM Cellular Dynamics Inc.
- RHEACELL GmbH And Co. KG.
- Takeda Pharmaceutical Company Limited.
- Teva Pharmaceutical Industries Ltd.
Report Scope:
In this report, the Global Stem Cell Manufacturing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Stem Cell Manufacturing Market, By Product:
- Consumables
- Instruments
- Stem Cell Lines
Stem Cell Manufacturing Market, By Application:
- Research Applications
- Clinical Application
- Cell and Tissue Banking Applications
Stem Cell Manufacturing Market, By End User:
- Pharmaceutical and Biotechnology Companies
- Academic Institutes
- Research Laboratories and Contract Research Organizations
- Hospitals and Surgical Centers
- Cell and Tissue Banks
- Others
Stem Cell Manufacturing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Stem Cell Manufacturing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific.
- Merck KGaA.
- AbbVie Inc.
- CO. LTD.
- Astellas Pharma Inc.
- Bristol-Myers Squibb Company.
- FUJIFILM Cellular Dynamics Inc.
- RHEACELL GmbH And Co. KG.
- Takeda Pharmaceutical Company Limited.
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | July 2025 |
| Forecast Period | 2024 - 2030 |
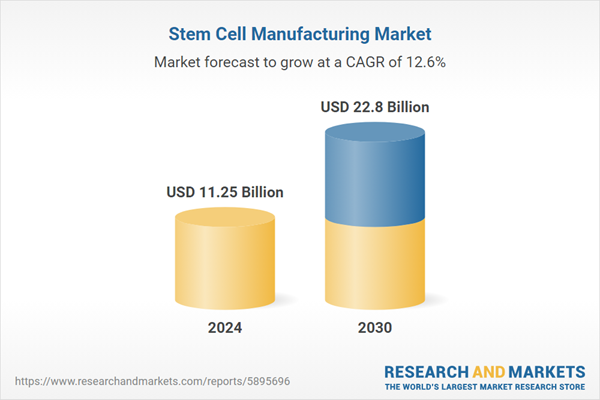
| Estimated Market Value ( USD | $ 11.25 Billion |
| Forecasted Market Value ( USD | $ 22.8 Billion |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |