Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Prevalence of Cardiovascular Diseases
The escalating incidence of cardiovascular diseases - such as coronary artery and peripheral arterial diseases - serves as a major catalyst for the artificial blood vessels market. This rise is driven by sedentary lifestyles, increased consumption of processed foods, and a surge in obesity and diabetes, all contributing to vascular complications. The global trend of aging populations further exacerbates vulnerability to arterial disorders due to natural physiological changes. According to the World Health Organization, cardiovascular diseases account for approximately 17.9 million deaths annually, highlighting the urgent need for advanced vascular solutions. A hereditary predisposition to heart disease and the health impacts of urbanization, including pollution and stress, also heighten the need for effective interventions like artificial grafts, thereby stimulating market growth.Key Market Challenges
Regulatory Hurdles and Approval Processes
One of the major challenges facing the artificial blood vessels market is navigating the stringent regulatory frameworks required for medical device approval. Compliance with rigorous standards from authorities such as the FDA and EMA involves lengthy and costly clinical evaluations, often delaying product launches. These regulatory procedures can vary significantly across regions, complicating global distribution strategies. Additionally, the focus on patient safety means that any adverse events or safety concerns can trigger intensified scrutiny, potential recalls, and prolonged approval timelines, thereby impeding market entry and expansion.Key Market Trends
Biodegradable and Bioengineered Materials
A significant trend in the artificial blood vessels market is the adoption of biodegradable and bioengineered materials that emulate the characteristics of natural vessels. These innovations enhance biocompatibility and minimize risks of clotting or immune reactions. Designed to gradually dissolve and integrate with patient tissue, these materials can help reduce long-term complications such as graft failure or calcification. Moreover, their customizable nature allows for tailored solutions that align with individual patient anatomies, improving fit and performance while reducing the risk of rejection, thus paving the way for personalized vascular care.Key Market Players
- B. Braun Melsungen
- Becton, Dickinson and Company
- Cook Medical Incorporated
- Humacyte Inc.
- Jotec GmbH
- LeMaitre Vascular Inc
- Medtronic Inc
- Techshot Inc
- Terumo Medical Corporation
- W. L. Gore & Associates, Inc.
Report Scope:
In this report, the Global Artificial Blood Vessels Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Artificial Blood Vessels Market, By Application:
- Aortic Disease
- Peripheral Artery Disease
- Haemodialysis
Artificial Blood Vessels Market, By Polymer:
- Polydioxanone
- Elastomer
- Polyethylene Terephthalate
- Others
Artificial Blood Vessels Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Artificial Blood Vessels Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- B. Braun Melsungen
- Becton, Dickinson and Company
- Cook Medical Incorporated
- Humacyte Inc.
- Jotec GmbH
- LeMaitre Vascular Inc
- Medtronic Inc
- Techshot Inc
- Terumo Medical Corporation
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
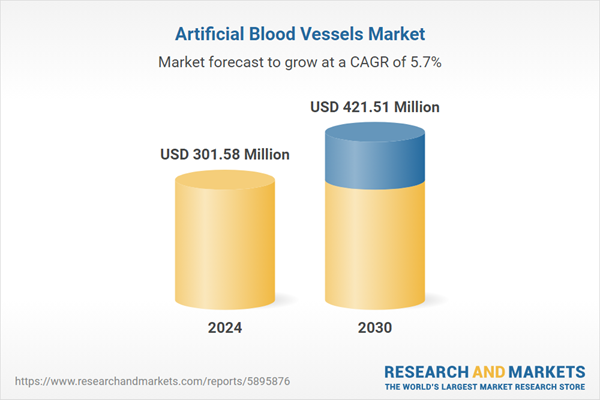
| Estimated Market Value ( USD | $ 301.58 Million |
| Forecasted Market Value ( USD | $ 421.51 Million |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |