Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these strong growth indicators, the market confronts significant obstacles due to the immense costs involved in the development and acquisition of these biologics. High production capital often results in elevated pricing structures, which limits accessibility in developing regions and places considerable strain on healthcare reimbursement systems. Consequently, strict pricing regulations and inadequate insurance coverage for premium immunotherapies persist as substantial barriers, potentially restricting the broader market penetration and commercial scalability of these advanced treatments.
Market Drivers
A primary market driver is the rapid evolution of antibody engineering, specifically the transition from standard monoclonal antibodies to highly potent antibody-drug conjugates (ADCs) and bispecific constructs. These advanced modalities enable the precise delivery of cytotoxic payloads to tumor cells while minimizing systemic toxicity, effectively addressing past efficacy limitations and resistance issues. This technological progression is triggering major strategic consolidations as companies aim to acquire proprietary platforms to strengthen their portfolios. For instance, Johnson & Johnson announced in March 2024 that it had finalized an all-cash merger valued at approximately $2.0 billion with Ambrx to integrate a suite of next-generation ADCs targeting metastatic prostate cancer and other solid tumors, underscoring the industry's shift toward complex biologics with superior therapeutic profiles.A second critical catalyst is the surging investment in oncology research and manufacturing infrastructure to support clinical pipelines and commercial supply chains. Pharmaceutical developers are dedicating substantial capital to establishing end-to-end production facilities to ensure the availability of these intricate drugs. A clear example of this is AstraZeneca's announcement in May 2024 regarding a capital expenditure of $1.5 billion to construct a greenfield manufacturing plant in Singapore dedicated to antibody-drug conjugates. This financial influx is heavily incentivized by the immense revenue potential of leading therapies; according to Merck & Co.'s February 2024 financial results, sales of their flagship antibody Keytruda grew by 19% to reach $25.0 billion, confirming the high return on investment available within this sector.
Market Challenges
The steep costs required to develop and acquire cancer monoclonal antibodies create a formidable barrier to the market's continued expansion. These biologic therapies involve complex manufacturing processes and rigorous clinical testing, resulting in high pricing structures that often exceed the financial capabilities of healthcare systems, particularly in developing nations. When payers and insurance providers face such elevated expenses, they frequently enforce strict reimbursement criteria or limit formulary inclusion. This restricts the patient population eligible for these life-saving treatments, directly reducing commercial volumes and hampering revenue growth in key international territories.Data from the International Society for Pharmacoeconomics and Outcomes Research in 2024 indicates that the cost for a single course of certain advanced immunotherapy regimens has reached approximately $318,000. Such elevated price points generate severe financial toxicity for patients and impose unsustainable burdens on national health budgets. Consequently, the market struggles to achieve broad penetration in cost-sensitive regions, as this economic pressure compels healthcare decision-makers to ration care or delay the adoption of premium biologic agents, effectively stalling the industry's potential momentum.
Market Trends
The market is currently experiencing a significant shift toward the commercialization of oncology biosimilars as patents for legacy monoclonal antibodies begin to expire. This trend enables healthcare systems to adopt cost-effective alternatives that preserve clinical efficacy while mitigating budget constraints, thereby broadening patient access to essential biologic therapies. Manufacturers are aggressively expanding their portfolios to capture market share in this high-volume segment, challenging the dominance of originator drugs. For example, Sandoz reported in its March 2024 results that net sales for its biopharmaceuticals business grew by 15% to reach USD 2.2 billion, a performance largely driven by the continued global uptake of its biosimilar assets.Simultaneously, pharmaceutical entities are prioritizing the development of subcutaneous formulations to replace traditional intravenous infusions for established cancer therapies. This reformulation strategy aims to alleviate the logistical burden on infusion centers and enhance patient convenience by drastically reducing the time required for treatment administration. By utilizing proprietary enzyme technologies to facilitate absorption, developers can deliver large-volume biologics via rapid injection rather than prolonged infusion. According to a September 2024 press release regarding the FDA approval of Genentech’s Tecentriq Hybreza, this newly approved subcutaneous option reduces administration time to approximately seven minutes, compared to the 30 to 60 minutes typically required for standard intravenous delivery.
Key Players Profiled in the Cancer Monoclonal Antibodies Market
- Amgen Inc.
- Bristol Myers Squibb Company
- Eli Lilly and Company
- Hoffmann-La Roche Ltd.
- Genmab AS
- GlaxoSmithKline PLC
- Johnson & Johnson
- Novartis AG
- Merck & Co., Inc.
- Spectrum Pharmaceuticals Inc.
Report Scope
In this report, the Global Cancer Monoclonal Antibodies Market has been segmented into the following categories:Cancer Monoclonal Antibodies Market, by Type of Monoclonal Antibody:
- Murine Antibodies
- Chimeric Antibodies
- Humanized Antibodies
Cancer Monoclonal Antibodies Market, by Monoclonal Antibody Therapies:
- Bevacizumab (Avastin)
- Rituximab (Rituxan)
- Trastuzumab (Herceptin)
- Cetuximab (Erbitux)
- Panitumumab (Vectibix)
- Other
Cancer Monoclonal Antibodies Market, by Application:
- Breast Cancer
- Blood Cancer
- Liver Cancer
- Brain Cancer
- Colorectal Cancer
- Other
Cancer Monoclonal Antibodies Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cancer Monoclonal Antibodies Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cancer Monoclonal Antibodies market report include:- Amgen Inc.
- Bristol Myers Squibb Company
- Eli Lilly and Company
- Hoffmann-La Roche Ltd
- Genmab AS
- GlaxoSmithKline PLC
- Johnson & Johnson
- Novartis AG
- Merck & Co., Inc
- Spectrum Pharmaceuticals Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
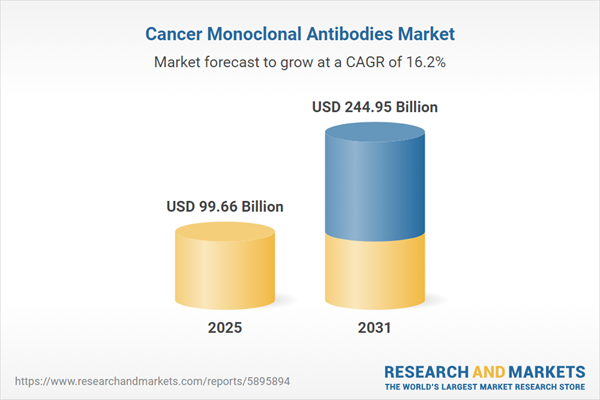
| Estimated Market Value ( USD | $ 99.66 Billion |
| Forecasted Market Value ( USD | $ 244.95 Billion |
| Compound Annual Growth Rate | 16.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |