Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Expanding Therapeutic Applications
The potential of iPSCs in clinical therapy is continuously growing as they are applied to an expanding spectrum of diseases. Their ability to differentiate into various specialized cell types makes them highly promising for treating complex conditions such as Parkinson’s disease, Alzheimer’s, cardiovascular disorders, and diabetes. iPSCs derived from adult cells can be engineered into dopaminergic neurons, offering targeted treatment pathways and facilitating drug development for neurodegenerative conditions. Similarly, their application in cardiac regeneration is gaining traction, with researchers using iPSC-derived cardiac cells to model heart diseases and explore regenerative options post-injury. The increasing adoption of iPSCs in these therapeutic areas not only enhances treatment potential but also broadens the market for their production, as healthcare systems and patients alike seek advanced, tailored solutions.Key Market Challenges
Cost of Production
A major limitation hindering the widespread adoption of iPSC technologies is the high cost associated with their generation and maintenance. The reprogramming of adult cells into iPSCs involves resource-intensive processes that require high-end laboratory infrastructure, advanced equipment, and skilled personnel. Furthermore, the culture media and reagents used in iPSC production are costly and must comply with strict quality standards to ensure consistency and safety. These operational expenses create significant financial barriers for both academic and commercial entities attempting to scale production for therapeutic use. Consequently, the cost of iPSC-based therapies remains high, limiting accessibility for broader patient groups and posing challenges for market scalability.Key Market Trends
Growing Applications in Disease Modeling and Drug Development
The increasing use of iPSCs in disease modeling and pharmaceutical research is a key trend driving market growth. iPSCs can be generated from individuals with specific genetic profiles, enabling the creation of personalized disease models. These models provide valuable platforms for understanding disease mechanisms and testing drug responses in conditions such as genetic disorders, neurodegenerative diseases, and cardiovascular anomalies. Pharmaceutical companies are leveraging iPSC technology to enhance drug discovery pipelines, enabling more accurate candidate screening and reducing development timelines and costs. The rising emphasis on precision medicine further amplifies the need for iPSC-derived models, reinforcing their significance in both academic research and commercial drug development.Key Market Players
- Lonza Group
- Axol Biosciences Ltd.
- Evotec SE
- Hitachi Ltd.
- Reprocells Inc.
- Fate Therapeutics.
- Thermo Fisher Scientific, Inc.
- Merck KgaA
- Stemcellsfactory III
- Applied Stemcells Inc.
Report Scope:
In this report, the Global Induced Pluripotent Stem Cells Production Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Induced Pluripotent Stem Cells Production Market, By Process:
- Manual iPSC Production Process
- Automated iPSC Production Process
Induced Pluripotent Stem Cells Production Market, By Product:
- Instruments/ Devices
- Automated Platforms
- Consumables & Kits
- Services
Induced Pluripotent Stem Cells Production Market, By End-user:
- Research & Academic Institutes
- Biotechnology & Pharmaceutical Companies
- Hospitals & Clinics
Induced Pluripotent Stem Cells Production Market, By Application:
- Drug Development and Discovery
- Regenerative Medicine
- Toxicology Studies
- Others
Induced Pluripotent Stem Cells Production Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Induced Pluripotent Stem Cells Production Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Lonza Group
- Axol Biosciences Ltd.
- Evotec SE
- Hitachi Ltd.
- Reprocells Inc.
- Fate Therapeutics.
- Thermo Fisher Scientific, Inc.
- Merck Kgaa
- Stemcellsfactory III
- Applied Stemcells Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
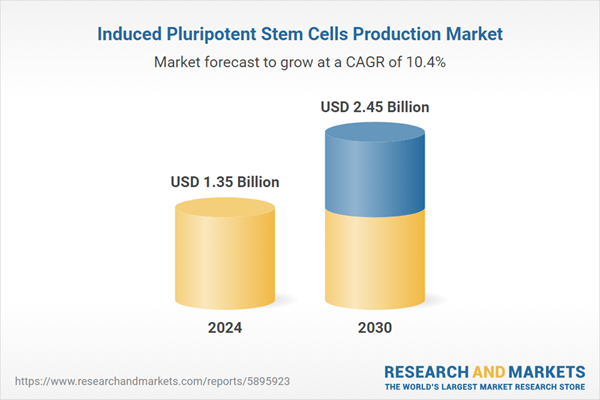
| Estimated Market Value ( USD | $ 1.35 Billion |
| Forecasted Market Value ( USD | $ 2.45 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |