Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Maternal and Fetal Safety
Maternal and fetal safety remains a critical driver of demand for intrapartum monitoring devices in global obstetric care. These devices play an essential role in continuously tracking vital maternal parameters such as blood pressure, heart rate, and oxygen saturation, enabling early detection of complications. Simultaneously, they monitor fetal heart rate and uterine contractions, offering real-time insights into the fetus’s condition. Any abnormalities in these readings allow healthcare providers to take immediate action, minimizing the risk of adverse outcomes during labor. The ability to ensure real-time surveillance and prompt medical intervention is central to reducing birth-related risks, making intrapartum monitoring indispensable for safeguarding both maternal and neonatal health during delivery.Key Market Challenges
Quality Assurance and Maintenance
Ensuring consistent performance and regulatory compliance of intrapartum monitoring devices poses a major challenge in the market. These devices must adhere to strict quality control protocols during manufacturing, including compliance with global standards like ISO 13485, to guarantee safety and accuracy. Beyond production, effective maintenance is crucial to uphold functionality. Healthcare providers must implement regular servicing, calibration, and inspection routines to prevent device failure during critical procedures. Inadequate maintenance or substandard manufacturing can compromise patient safety, emphasizing the importance of robust quality assurance and lifecycle management across the healthcare ecosystem.Key Market Trends
Remote Monitoring and Telehealth
The emergence of remote monitoring and telehealth solutions is transforming the intrapartum monitoring landscape. These technologies enable healthcare providers to oversee maternal and fetal well-being from a distance through connected monitoring devices that transmit real-time data. Remote capabilities are particularly valuable for managing high-risk pregnancies and serving populations in remote or underserved regions. Telehealth platforms further enhance care delivery by facilitating consultations and continuous guidance during labor, reducing the need for in-person visits. This digital evolution enhances accessibility, ensures timely interventions, and minimizes healthcare costs, making remote monitoring and telehealth integral trends in improving maternal and fetal outcomes worldwide.Key Market Players
- Cardinal Health
- GE Healthcare
- Koninklijke Philips N.V.
- MindChild Medical
- The Cooper Companies, Inc.
- General Electric Company
- Huntleigh Healthcare Limited
- MedGyn products, Inc.
- Rocket Medical plc
- Stalwart Meditech
Report Scope:
In this report, the Global Intrapartum Monitoring Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Intrapartum Monitoring Devices Market, By Product Type:
- Electrodes
- Monitors
Intrapartum Monitoring Devices Market, By End User:
- Hospitals
- Maternity Centers
- Others
Intrapartum Monitoring Devices Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Intrapartum Monitoring Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional Market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Cardinal Health
- GE Healthcare
- Koninklijke Philips N.V.
- MindChild Medical
- The Cooper Companies, Inc.
- General Electric Company
- Huntleigh Healthcare Limited
- MedGyn products, Inc.
- Rocket Medical PLC
- Stalwart Meditech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
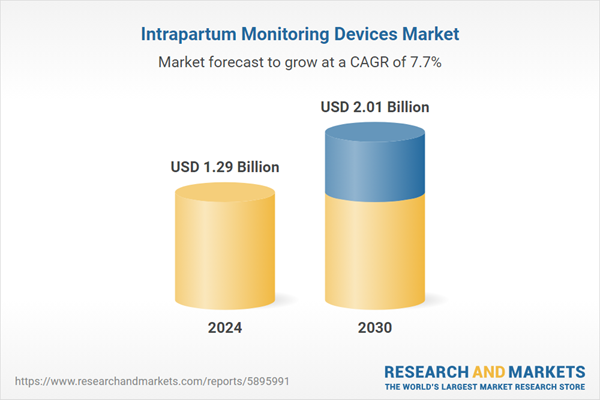
| Estimated Market Value ( USD | $ 1.29 Billion |
| Forecasted Market Value ( USD | $ 2.01 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |