Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
This market includes a broad spectrum of sophisticated medical devices and integrated systems that enable the real-time monitoring of vital signs such as heart rate, respiratory rate, blood pressure, oxygen saturation, temperature, and other critical physiological parameters. These monitoring solutions are widely utilized in hospitals, ambulatory care centers, home care settings, and intensive care units, supporting healthcare professionals in making timely and accurate clinical decisions. For instane, in 2024, BROADSIMS introduced a 5G smart patient monitoring solution aimed at building a connected healthcare ecosystem. This innovation enhances real-time data transmission, streamlines care delivery, and significantly improves healthcare efficiency across various medical settings.
Key Market Drivers
The Role of Technology Advancements
Technology advancements are at the forefront of driving the Global Multi-Parameter Patient Monitoring Market. These innovations have redefined the landscape of healthcare by introducing cutting-edge solutions that enhance patient care and streamline medical processes. For instance, in January 2023, Biobeat - a global leader in wearable remote patient monitoring devices, announced that it had received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This approval enables the company to monitor a range of vital health indicators, including stroke volume, cardiac output, cuffless blood pressure, oxygen saturation, pulse rate, respiratory rate, and body temperature.Miniaturized, high-precision sensors have become the backbone of patient monitoring. These sensors can capture a wide range of vital signs and health parameters with remarkable accuracy. The integration of wireless technology enables real-time data transmission from monitoring devices to healthcare providers' systems. This seamless data flow ensures that medical professionals can access critical patient information promptly, even from remote locations. Sophisticated data analytics tools process the wealth of information collected by multi-parameter patient monitoring systems.
These tools help in identifying trends, anomalies, and potential issues, enabling healthcare providers to make informed decisions quickly. Wearable monitoring devices have gained popularity among patients and healthcare professionals alike. These compact and user-friendly gadgets allow patients to monitor their health continuously, providing valuable insights into their well-being. The advent of remote monitoring has transformed patient care. Physicians can now track their patients' vital signs and health status outside traditional hospital settings, reducing hospital readmissions and improving overall patient outcomes.
Key Market Challenges
Data Security and Privacy Concerns
As multi-parameter patient monitoring systems collect and transmit sensitive health data, concerns about data security and patient privacy are paramount. Several challenges related to data security and privacy can pose obstacles to market expansion:The healthcare industry is a prime target for cyberattacks and data breaches. A breach of patient health data can have severe legal, financial, and reputational consequences for healthcare providers and device manufacturers. Healthcare data is subject to strict regulatory frameworks, such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States. Compliance with these regulations requires significant investment in data security measures and compliance monitoring. Patients must have confidence that their health data is secure and that their privacy is protected. Any perception of insecurity can hinder the adoption of multi-parameter patient monitoring systems. Addressing these security and privacy concerns is essential for the market to gain widespread acceptance and trust among healthcare providers and patients.
Key Market Trends
Remote Patient Monitoring (RPM)
Remote Patient Monitoring is a transformative trend in the healthcare industry that has gained significant traction in the Global Multi-Parameter Patient Monitoring Market. This trend involves the use of advanced monitoring devices to track patients' vital signs and health parameters from the comfort of their homes or other non-hospital settings. Key aspects of this trend include:RPM allows healthcare providers to monitor patients continuously without the need for frequent in-person visits. This is especially beneficial for patients in remote areas or those with limited mobility. RPM is particularly valuable for individuals with chronic diseases, such as diabetes, hypertension, and heart conditions. These patients can receive timely interventions and adjustments to their treatment plans based on real-time data, reducing the risk of complications. As the global population ages, RPM provides a means to monitor the health of elderly individuals more effectively, allowing them to maintain independence and age in place. The data collected through RPM is often analyzed using advanced analytics tools, enabling healthcare providers to detect trends and potential issues early, thus facilitating proactive care.
Key Market Players
- Abbott Laboratories
- Baxter International Inc.
- Becton, Dickinson and Company
- Boston Scientific Corporation
- General Electric Company (GE Healthcare)
- Medtronic PLC
- Koninklijke Philips NV
- SCHILLER
- Nihon Kohden
Report Scope:
In this report, the Global Multi-Parameter Patient Monitoring Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Multi-Parameter Patient Monitoring Market, By Device Type:
- Portable
- Fixed
Multi-Parameter Patient Monitoring Market, By Acuity Level:
- High Acuity Level
- Medium Acuity Level
- Low Acuity Level
Multi-Parameter Patient Monitoring Market, By Target Area:
- Cardiology
- Neurology
- Respiratory
- Fetal and Neonatal
- Temperature Monitoring
- Other
Multi-Parameter Patient Monitoring Market, By End-User:
- Hospitals
- Home Healthcare
Multi-Parameter Patient Monitoring Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Multi-Parameter Patient Monitoring Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Baxter International Inc.
- Becton, Dickinson and Company
- Boston Scientific Corporation
- General Electric Company (GE Healthcare)
- Medtronic PLC
- Koninklijke Philips NV
- SCHILLER
- Nihon Kohden
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | June 2025 |
| Forecast Period | 2024 - 2030 |
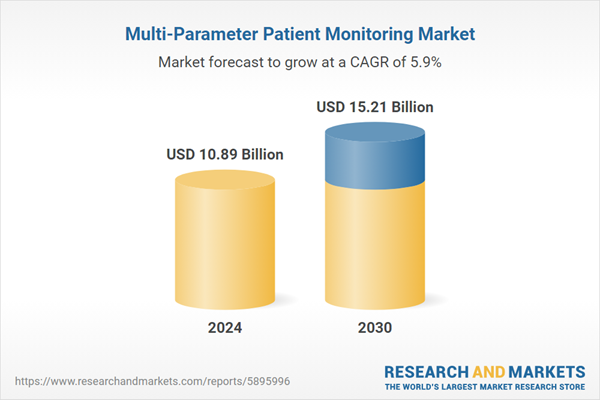
| Estimated Market Value ( USD | $ 10.89 Billion |
| Forecasted Market Value ( USD | $ 15.21 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |