Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
As clinical trials become more globalized and data-intensive - incorporating vast datasets from diverse sources such as electronic health records (EHRs), genomic sequences, and medical histories - there is a growing reliance on intelligent software platforms that can efficiently match patients to appropriate studies. These platforms have become a critical operational tool in overcoming the logistical and financial challenges associated with clinical research.
Patient recruitment remains one of the most significant hurdles in clinical trials, often causing delays and budget overruns. Manual identification of eligible participants is typically time-consuming and error-prone. Clinical trials matching software addresses this issue by leveraging advanced technologies such as artificial intelligence (AI) and machine learning (ML). These systems analyze both structured and unstructured patient data to accurately identify candidates that meet complex inclusion and exclusion criteria, thereby accelerating recruitment timelines and increasing trial success rates.
Key Market Drivers
Rising Demand for Personalized Medicine
Personalized medicine is transforming the healthcare landscape by enabling tailored treatment strategies based on an individual’s genetic profile, lifestyle, and specific health conditions. This approach is not only enhancing patient outcomes but also reshaping the pharmaceutical research model. As the demand for personalized therapies grows, it is fueling an increase in clinical trial activity and driving the need for advanced patient-matching technologies.Also referred to as precision medicine, this approach moves away from the traditional “one-size-fits-all” treatment model and focuses on individualized care informed by genetic, molecular, and environmental factors. It has shown particular success in oncology, where therapies are developed to target specific genetic mutations, resulting in more effective and less toxic treatments.
The implementation of personalized medicine requires in-depth genetic profiling and biomarker identification, generating vast amounts of complex data that must be analyzed and validated through clinical trials. Clinical trials matching software is pivotal in managing this data and accelerating the recruitment of appropriate trial participants, ensuring the timely execution of research and improving the likelihood of clinical success.
Key Market Challenges
Data Privacy and Security
One of the primary challenges confronting the clinical trials matching software market is the management of data privacy and security. These platforms handle highly sensitive information, including patients’ medical records, genetic data, and other personal health details. Ensuring compliance with stringent data protection regulations - such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union - is both complex and resource-intensive.Failure to adhere to these regulations can result in legal repercussions, reputational damage, and loss of stakeholder trust. As a result, software developers must prioritize robust security frameworks and ensure their platforms are fully compliant with global data protection standards.
Key Market Trends
Integration of Artificial Intelligence and Machine Learning
The adoption of artificial intelligence (AI) and machine learning (ML) technologies is emerging as a major trend in the clinical trials matching software market. These technologies are enhancing the ability of software platforms to process large volumes of data, identify eligible trial candidates, and predict patient responses to investigational therapies.ML-powered tools not only improve recruitment efficiency but also support smarter trial design and real-time monitoring, which ultimately accelerates drug development timelines. As AI capabilities continue to evolve, their integration into clinical trial workflows is expected to redefine how trials are conducted, from protocol design to patient engagement and outcome analysis.
Key Market Players
- IBM Clinical Development
- Antidote Technologies Inc
- Ofni Systems Inc
- SSS International Clinical Research GmbH
- CLARIO
- Advarra Inc
- ArisGlobal LLC
- Bsi Business Systems Integration AG
- Teckro Ltd
- Clinical Trials Mobile Application
Report Scope:
In this report, the Global Clinical Trials Matching Software Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Clinical Trials Matching Software Market, By Deployment Mode:
- Web & Cloud-based
- On-premises
Clinical Trials Matching Software Market, By End-use:
- Pharmaceutical & Biotechnology Companies
- CROs
- Medical Device Firms
Clinical Trials Matching Software Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Clinical Trials Matching Software Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- IBM Clinical Development
- Antidote Technologies Inc
- Ofni Systems Inc
- SSS International Clinical Research GmbH
- CLARIO
- Advarra Inc
- ArisGlobal LLC
- Bsi Business Systems Integration AG
- Teckro Ltd
- Clinical Trials Mobile Application
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
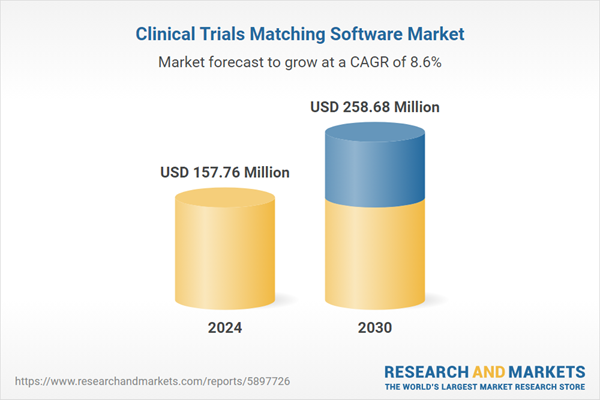
| Estimated Market Value ( USD | $ 157.76 Million |
| Forecasted Market Value ( USD | $ 258.68 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |