Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive indicators, patient recruitment remains a formidable challenge that threatens to slow market growth. The restricted number of eligible participants, combined with rigorous ethical standards regarding informed consent, frequently results in prolonged timelines and escalating costs. Consequently, the difficulty in securing adequate enrollment to produce statistically robust data serves as a persistent obstacle for pharmaceutical companies and contract research organizations, potentially hindering the timely development of pediatric therapies.
Market Drivers
The broadening of financial incentives and market exclusivity provisions serves as a major catalyst for market expansion, particularly through initiatives such as the Rare Pediatric Disease Priority Review Voucher (PRV) program. These regulatory mechanisms transform successful pediatric drug development into valuable commercial assets, encouraging developers to target smaller, often less profitable patient groups. The monetization potential of these incentives has surged recently, fostering a competitive secondary market for vouchers that helps subsidize high research costs. BioSpace reported in March 2025 that the trading price for these vouchers reached $150 million early in the year, driven by demand from major pharmaceutical firms seeking expedited review for flagship products, thereby providing immediate capital to smaller biotech firms and reducing financial risks.Simultaneously, a significant increase in R&D for rare and orphan pediatric disorders is reshaping the clinical landscape, as the industry leverages the higher regulatory success rates associated with these special designations. Companies are increasingly prioritizing niche indications with high unmet needs, utilizing the fact that orphan-designated therapies often navigate approval processes more efficiently than standard treatments. According to Nome Bio in November 2025, orphan-designated products are achieving approval rates of 25-30%, significantly outperforming the 10-12% success rate of conventional drugs. This strategic focus is reflected in data from the Pharmaceutical Research and Manufacturers of America (PhRMA), which noted in October 2025 that over 2,100 industry-sponsored pediatric trials involving 1.2 million patients are currently underway, highlighting the sector's commitment to complex pediatric conditions.
Market Challenges
Difficulties regarding patient recruitment significantly hinder the growth of the Global Pediatric Clinical Trials Market by causing operational bottlenecks that delay study completion and increase development costs. The scarcity of eligible pediatric subjects, exacerbated by strict ethical requirements for informed consent, often leads to trials failing to meet enrollment targets within planned schedules. When pharmaceutical companies are unable to gather sufficient data promptly, the entire development pipeline slows, which discourages future investment in pediatric-specific indications and limits overall market volume.This operational inefficiency is supported by recent industry findings. Data from the Association of Clinical Research Professionals indicates that in 2024, 36% of clinical research sites cited patient recruitment and retention as their primary operational challenge. This issue is even more acute in pediatric research, where patient pools are naturally smaller and more geographically dispersed than in adult populations. Consequently, the inability to efficiently enroll participants directly impedes market growth by extending trial durations and increasing financial risks for sponsors, resulting in a more cautious approach toward initiating new pediatric studies.
Market Trends
The adoption of Artificial Intelligence for Precise Cohort Identification is fundamentally transforming patient selection strategies by moving from manual screening to the algorithmic analysis of electronic health records. This technological integration enables sponsors to swiftly identify eligible pediatric participants by processing unstructured clinical notes and genetic markers that traditional methods might miss. By automating the detection of specific inclusion criteria across vast datasets, pharmaceutical developers can alleviate operational delays caused by the shortage of eligible subjects. A June 2024 report by the National Institutes of Health, titled 'Applying Artificial Intelligence in Pediatric Clinical Trials,' noted that AI algorithms used in pediatric oncology trials reduced the patient screening workload by up to 90%, significantly accelerating the identification of suitable candidates.In parallel, the increasing use of Wearable Devices for Remote Pediatric Monitoring is enhancing data integrity and participant retention by minimizing the need for intrusive on-site visits. These non-invasive tools allow for the continuous collection of physiological biomarkers in the child's natural environment, thereby reducing the logistical burden on families and ensuring consistent engagement during long-term studies. This shift improves the quality of real-world evidence gathered and supports a patient-centric approach aligned with the needs of pediatric populations. According to the Association of Clinical Research Professionals in October 2024, in the 'Enhancing Clinical Trials with Wearable Digital Health Technologies' article, trials incorporating wearable technologies achieved high patient adherence rates between 70% and 80%, demonstrating their effectiveness in maintaining protocol compliance.
Key Players Profiled in the Pediatric Clinical Trials Market
- Bristol-Myers Squibb Company
- Charles River Laboratories International Inc.
- Covance Inc.
- GlaxoSmithKline PLC
- ICON PLC
- IQVIA Inc.
- Novartis AG
- Pfizer, Inc.
- Pharmaceutical Product Development, LLC
- Syneos Health Inc.
- Paidion Research, Inc.
- The Emmes Company, LLC
Report Scope
In this report, the Global Pediatric Clinical Trials Market has been segmented into the following categories:Pediatric Clinical Trials Market, by Phase:
- Phase I
- Phase II
- Phase III
- and Phase IV
Pediatric Clinical Trials Market, by Study Design:
- Treatment Studies and Observational Studies
Pediatric Clinical Trials Market, by Therapeutic Area:
- Respiratory Diseases
- Infectious Diseases
- Oncology
- Diabetes
- and Other Therapeutic Areas
Pediatric Clinical Trials Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Pediatric Clinical Trials Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Pediatric Clinical Trials market report include:- Bristol-Myers Squibb Company
- Charles River Laboratories International Inc.
- Covance Inc.
- GlaxoSmithKline PLC
- ICON PLC
- IQVIA Inc.
- Novartis AG
- Pfizer, Inc.
- Pharmaceutical Product Development, LLC
- Syneos Health Inc.
- Paidion Research, Inc.
- The Emmes Company, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
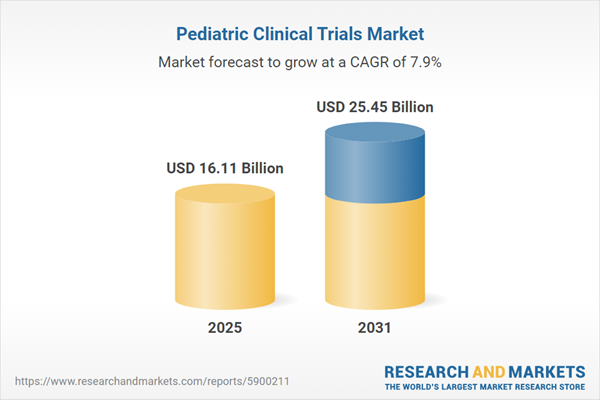
| Estimated Market Value ( USD | $ 16.11 Billion |
| Forecasted Market Value ( USD | $ 25.45 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |