Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
This accumulation leads to a wide range of symptoms and organ damage, affecting multiple systems in the body. Most LSDs result from deficiencies of specific lysosomal enzymes. These enzymes are responsible for breaking down complex molecules, such as lipids (fats), glycoproteins, and mucopolysaccharides. When a particular enzyme is deficient or absent, the corresponding substrate accumulates within lysosomes. There are over 50 different LSDs, each associated with a specific lysosomal enzyme deficiency. Examples of LSDs include Gaucher's disease, Tay-Sachs disease, Fabry disease, Pompe disease, Niemann-Pick disease, and mucopolysaccharidoses (MPS). Each LSD has its unique clinical features and disease course. For instance, according to the National Institutes of Health (NIH), as of April 2022, Pompe disease affects approximately 1 in 40,000 individuals in the United States.
Key Market Drivers
Advances in Research and Development
Gene therapy has emerged as a promising approach for treating certain LSDs. Researchers are exploring methods to deliver functional genes into affected cells to restore enzyme production. Clinical trials and studies have shown promising results for diseases like mucopolysaccharidosis type II (Hunter syndrome) and Niemann-Pick disease type A and B. According to a July 2022 study published by the National Library of Medicine, lysosomal storage disorders (LSDs) are more prevalent when considered collectively, with a combined incidence of 1 in 5,000 to 1 in 8,000.The study highlights that ethnicity and geography influence LSD occurrence. For instance, Gaucher disease (GD) affects 1 in 40,000 to 1 in 60,000 in the general population but is significantly higher among Ashkenazi Jews (1 in 800). Similarly, Tay-Sachs disease (1 in 3,900), Niemann-Pick A, and mucolipidosis IV are more common in this group. In Finland, aspartylglucosaminuria occurs in 1 in 18,500 individuals. The increasing burden of LSDs is expected to drive market growth for lysosomal storage disease treatments in the coming years.
Small molecule chaperones are designed to stabilize mutant enzymes, allowing them to function more effectively. These therapies aim to correct the underlying enzymatic defects in LSDs. Some chaperone therapies have received regulatory approval and are available for patients with conditions like Fabry disease. Substrate Reduction Therapy (SRT) involves reducing the production of the toxic substrate that accumulates in lysosomes in LSDs. Medications like miglustat and eliglustat have been developed as SRTs and are used to treat diseases such as Gaucher's disease and Niemann-Pick type C. Ongoing research has led to the development of improved Enzyme Replacement Therapy (ERTs) with enhanced stability, bioavailability, and pharmacokinetics.
These advancements aim to increase the effectiveness and convenience of treatment for patients with LSDs. Researchers are investigating the potential benefits of combining different therapeutic approaches, such as ERTs with chaperone therapies or gene therapy with small molecules. These combinations may offer synergistic effects and improved patient outcomes. Advances in biomarker research have led to the identification of specific markers that can aid in the diagnosis, monitoring, and assessment of disease progression in LSDs.
Key Market Challenges
Limited Understanding of Disease Mechanisms
In many LSDs, the underlying genetic and molecular mechanisms are complex and not fully understood. This complexity makes it challenging to develop targeted therapies that address the root cause of the disease. Without a comprehensive understanding of the disease mechanisms, it is difficult to identify specific drug targets and design effective treatments. LSDs encompass a wide range of rare genetic disorders, each with its unique pathophysiology. Understanding the variations in disease mechanisms among different LSDs is essential for developing tailored treatments. Limited knowledge of these variations can hinder therapeutic development efforts. Biomarkers are crucial for disease diagnosis, monitoring, and assessing treatment efficacy.However, without a deep understanding of disease mechanisms, it can be challenging to identify reliable biomarkers for LSDs, which are necessary for clinical trials and personalized medicine approaches. The lack of insight into disease mechanisms contributes to a high failure rate in drug development for LSDs. Many potential drug candidates do not progress past preclinical or early clinical stages because they do not effectively target the underlying disease processes.
In the absence of a clear understanding of disease mechanisms, drug developers may face challenges related to off-target effects. These unintended consequences can lead to safety concerns and hinder the development of safe and effective therapies. The complexity of LSDs and the limited understanding of their mechanisms can make it difficult to secure research funding. Potential investors and grant providers may be hesitant to fund projects without a clear path to success, leading to underfunding of critical research efforts.
Key Market Trends
Chaperone Therapies
Chaperone therapies involve the use of small molecules that can stabilize and enhance the activity of misfolded or unstable lysosomal enzymes in LSDs. These molecules act as chaperones by assisting in the correct folding and trafficking of the enzyme to its target location within the lysosome. Chaperone therapies are designed to address the specific genetic mutations that lead to enzyme misfolding and dysfunction in LSDs. They target the underlying cause of the disease by helping the enzyme reach its active form, which is essential for substrate degradation. Many chaperone therapies are administered orally, which is a more convenient and patient-friendly route of administration compared to intravenous infusions or other invasive methods.This can improve treatment adherence and patient quality of life. Chaperone therapies have been developed and tested for various LSDs, including Fabry disease, Pompe disease, Gaucher's disease, and others. This broad applicability makes them relevant to multiple LSD subtypes. Some chaperone therapies have demonstrated clinical success and received regulatory approvals in different regions. For example, migalastat has been approved for the treatment of Fabry disease.
Chaperone therapies hold the potential to modify the course of the disease by restoring enzyme activity and reducing substrate accumulation. This can lead to improvements in clinical outcomes and the prevention of disease progression. Researchers are exploring the possibility of combining chaperone therapies with other treatment approaches, such as enzyme replacement therapy (ERT) or gene therapy. These combination therapies may offer synergistic benefits and enhanced treatment efficacy. Chaperone therapies align with the trend toward personalized and precision medicine, where treatments are tailored to individual patients based on their specific genetic mutations and disease manifestations.
Key Market Players
- Pfizer, Inc.
- Sanofi SA
- BioMarin Pharmaceutical Inc
- Actelion Ltd.
- Raptor Pharmaceutical Corp.
- Protalix Biotherapeutics Inc.
- Amicus Therapeutics, Inc.
- Quest Diagnostics Inc.
- Amicus Therapeutics Inc.
- Shire Plc
Report Scope:
In this report, the Global Lysosomal Storage Diseases Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Lysosomal Storage Diseases Therapeutics Market, By Treatment:
- Enzyme Replacement Therapy
- Stem Cell Therapy
- Substrate Reduction Therapy
- Others
Lysosomal Storage Diseases Therapeutics Market, By Indication:
- Gaucher's Disease
- Fabry Disease
- Pompe's Syndrome
- Mucopolysaccharidosis
- Others
Lysosomal Storage Diseases Therapeutics Market, By End-User:
- Hospitals
- Clinics
Lysosomal Storage Diseases Therapeutics Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Lysosomal Storage Diseases Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer, Inc.
- Sanofi SA
- BioMarin Pharmaceutical Inc
- Actelion Ltd.
- Raptor Pharmaceutical Corp.
- Protalix Biotherapeutics Inc.
- Amicus Therapeutics, Inc.
- Quest Diagnostics Inc.
- Amicus Therapeutics Inc.
- Shire Plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
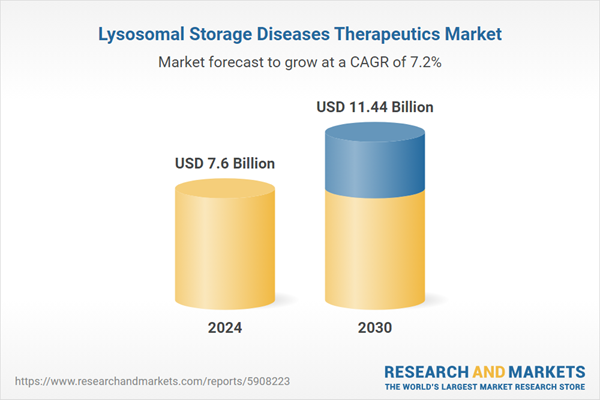
| Estimated Market Value ( USD | $ 7.6 Billion |
| Forecasted Market Value ( USD | $ 11.44 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |