Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive indicators, the market confronts a major obstacle regarding widespread consumer dependence on over-the-counter laxatives and dietary supplements. A substantial number of individuals experiencing symptoms opt for self-medication with affordable, accessible alternatives rather than pursuing professional medical diagnoses, a behavior that postpones the shift to advanced prescription therapies. This tendency significantly constrains the addressable audience for specialized chronic idiopathic constipation therapeutics and hinders broader revenue growth within the sector.
Market Drivers
The rising global incidence of chronic idiopathic constipation, fueled by sedentary lifestyles and dietary modifications, serves as a primary engine for market expansion. As urbanization accelerates and physical activity diminishes, the prevalence of functional bowel disorders has increased, resulting in a larger patient population requiring medical care. This dynamic has led to a noticeable shift of patients moving from over-the-counter remedies to prescription-grade treatments, creating consistent demand for established therapies. Recent industry metrics reflect this trend; according to Ironwood Pharmaceuticals' Third Quarter 2025 Results released in December 2025, total prescription demand for LINZESS rose by 12% compared to the prior year.Revenue growth is further stimulated by pharmacological innovations and the introduction of therapies with novel mechanisms of action, which offer enhanced efficacy for difficult-to-treat cases. The sector is evolving toward next-generation agents, including sodium-hydrogen exchanger 3 (NHE3) inhibitors and guanylate cyclase-C agonists, which are quickly capturing market share.
This adoption is evidenced by financial performance; Ardelyx reported in its October 2025 Third Quarter Financial Results that net product sales for the novel agent IBSRELA hit $78.2 million due to increased prescriber uptake. Additionally, the significant commercial value of these specialized treatments is highlighted by Ironwood Pharmaceuticals' February 2025 report, which noted that full-year 2024 U.S. net sales for LINZESS alone reached $916.3 million.
Market Challenges
A major hurdle inhibiting the Global Chronic Idiopathic Constipation Therapeutic Market is the extensive patient reliance on dietary supplements and over-the-counter laxatives. This deeply rooted habit of self-medication establishes a significant barrier to prescription therapy adoption, as individuals usually utilize affordable, readily available retail products before seeking professional advice. Consequently, formal diagnoses of Chronic Idiopathic Constipation are often delayed or overlooked, effectively preventing a substantial segment of the potential patient base from accessing advanced, higher-value prescription treatments that generate market revenue.This hesitation to pursue medical intervention limits the reach of specialized therapeutics, even when severe symptoms warrant professional care. Pharmaceutical manufacturers face difficulties in gaining market share when patients view non-prescription alternatives as sufficient, regardless of their actual long-term effectiveness. For instance, the American College of Gastroenterology reported in a 2024 real-world survey that 95% of patients with constipation-related issues experienced straining during bowel movements, indicating a heavy symptom burden that remains despite self-care attempts. This gap between high symptom severity and persistent dependence on retail solutions curtails the uptake of prescription therapies, directly impeding the market's financial growth.
Market Trends
Pharmaceutical companies are increasingly expanding label indications to include pediatric populations, thereby widening the addressable patient base for chronic idiopathic constipation therapeutics. Manufacturers are securing regulatory approvals to extend the use of established guanylate cyclase-C agonists to adolescents and children, a group previously restricted to osmotic laxatives or off-label treatments. This strategic development targets a significant unmet need within pediatric functional bowel disorders while validating safety profiles for younger users. As reported by Pharmaceutical Technology in November 2025, the FDA approved Linzess for children aged seven and older with irritable bowel syndrome with constipation, effectively expanding the drug's market reach beyond its original adult indications.Concurrently, the competitive landscape is being reshaped by the introduction of generic prokinetic agents, which are lowering cost barriers. The patent expiration for serotonin-4 receptor agonists has prompted the release of bioequivalent alternatives, making treatments more accessible to patients limited by the high out-of-pocket expenses associated with branded drugs. This availability of lower-cost options enables healthcare providers to prescribe specialized prokinetics more frequently, boosting volume growth in this segment. For example, ANI Pharmaceuticals announced in January 2025 the launch of the first U.S. generic version of Motegrity (prucalopride), targeting a branded market estimated at $168 million annually.
Key Players Profiled in the Chronic Idiopathic Constipation Therapeutic Market
- GSK PLC
- Astellas Pharma Inc.
- Synergy Pharmaceuticals Inc.
- Bayer AG
- Allergan PLC
- Sanofi SA
- Pfizer Inc.
- Takeda Pharmaceutical Co Ltd.
- Zydus Lifesciences Ltd.
- Boehringer Ingelheim GmbH
Report Scope
In this report, the Global Chronic Idiopathic Constipation Therapeutic Market has been segmented into the following categories:Chronic Idiopathic Constipation Therapeutic Market, by Drug Class:
- Bulk-Forming Agents
- Emollients
- Laxatives
- Osmotic Agents
Chronic Idiopathic Constipation Therapeutic Market, by Route of Administration:
- Oral
- Parenteral
Chronic Idiopathic Constipation Therapeutic Market, by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Chronic Idiopathic Constipation Therapeutic Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Chronic Idiopathic Constipation Therapeutic Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Chronic Idiopathic Constipation Therapeutic market report include:- GSK PLC
- Astellas Pharma Inc
- Synergy Pharmaceuticals Inc.
- Bayer AG
- Allergan PLC
- Sanofi SA
- Pfizer Inc
- Takeda Pharmaceutical Co Ltd
- Zydus Lifesciences Ltd
- Boehringer Ingelheim GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
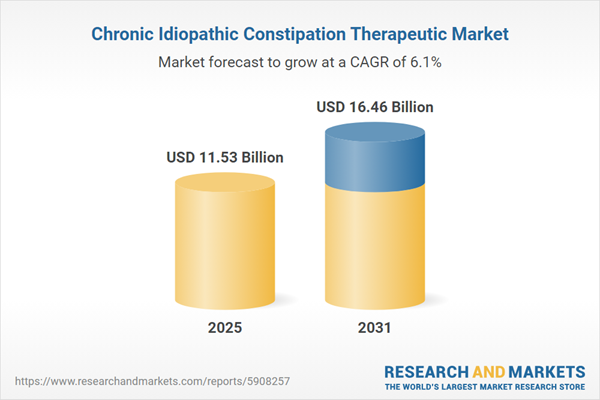
| Estimated Market Value ( USD | $ 11.53 Billion |
| Forecasted Market Value ( USD | $ 16.46 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |