Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces substantial challenges regarding manufacturing complexity and production scalability. Synthesizing bispecific antibodies involves intricate processes to ensure correct chain pairing and stability, often resulting in lower yields and higher costs compared to conventional monoclonal antibodies. These technical hurdles make upscaling production difficult and necessitate stringent quality control measures. Such requirements can delay regulatory clearance and inflate the final cost of therapy, potentially impeding widespread market accessibility.
Market Drivers
Strategic collaborations and licensing agreements within the biotechnology sector are fundamentally reshaping the landscape as pharmaceutical giants aggressively acquire external innovation to strengthen their immuno-oncology portfolios. Major players are mitigating early-stage development risks by purchasing established bispecific platforms capable of targeting multiple disease pathways simultaneously. This trend fosters an ecosystem where smaller biotechs receive essential capital while larger firms secure high-potential candidates for their pipelines. For example, according to Fierce Biotech, Merck entered a definitive agreement in January 2024 to acquire Harpoon Therapeutics for approximately $680 million to access its proprietary T-cell engager technology.A surge in regulatory approvals for novel bispecific therapies further drives the market, validating the safety and efficacy of these complex modalities for broader patient populations. As manufacturing and clinical protocols standardize, regulatory bodies are accelerating authorizations, moving these treatments from experimental stages to mainstream practice. The Antibody Society noted that in 2024, agencies granted first approvals to several new bispecifics, including tarlatamab and zanidatamab. This regulatory success supports commercial viability, as evidenced by Roche’s report in October 2024 that sales of its bispecific medicine Vabysmo reached CHF 2.8 billion in the first nine months of the year.
Market Challenges
The complexity of manufacturing and production scalability remains a significant barrier to the expansion of the Global Bispecific Antibodies Market. Unlike standard monoclonal antibodies, bispecific formats require the precise engineering of two distinct binding arms, a process that inherently increases the risk of chain mispairing and product instability. These technical intricacies frequently result in lower production yields and higher levels of impurities, which directly escalate the cost of goods sold. Consequently, manufacturers face difficulties in upscaling processes to commercial levels, leading to high pricing structures that limit therapy accessibility and market penetration.Moreover, these production hurdles necessitate stringent quality control measures that extend development timelines and delay regulatory decision-making. The rigorous scrutiny required to ensure the stability and safety of these complex molecules contributes to a high attrition rate during late-stage development. According to The Antibody Society, the overall approval success rates for antibody therapeutics in 2024 were estimated to range between 14% and 32%, reflecting the substantial developmental risks and regulatory hurdles that impede the rapid commercialization of such complex modalities and slow the market's growth trajectory.
Market Trends
The market is experiencing a definitive shift towards subcutaneous delivery formulations, driven by the need to enhance patient convenience and optimize healthcare resource utilization. Manufacturers are actively re-engineering intravenous therapies into subcutaneous formats to significantly reduce chair time and administration complexity, addressing the logistical burdens of chronic treatment regimens. This trend is supported by clinical evidence; according to Johnson & Johnson, data from the Phase 3 PALOMA-3 study in May 2024 demonstrated that the subcutaneous formulation of amivantamab reduced infusion-related reactions five-fold compared to the intravenous version while shortening administration time to approximately five minutes.Concurrently, there is a major trend regarding the diversification of indications beyond oncology, as pharmaceutical developers pivot these modalities toward high-prevalence autoimmune disorders. While oncology remains a key area, the ability of bispecific antibodies to precisely deplete pathogenic B-cells or modulate dual inflammatory pathways has triggered increased investment in non-cancer applications such as atopic dermatitis and rheumatoid arthritis. This strategic expansion is exemplified by Merck & Co.’s agreement in August 2024 to acquire Curon Biopharmaceutical’s clinical-stage bispecific antibody CN201 for a potential $1.3 billion, explicitly aiming to leverage the asset for treating B-cell associated autoimmune diseases.
Key Players Profiled in the Bispecific Antibodies Market
- Amgen Inc.
- F. Hoffmann-La Roche Ltd.
- Genentech Inc.
- Akeso Inc.
- Johnson & Johnson Private Limited
- Taisho Pharmaceutical Co Ltd.
- Immunocore Ltd.
Report Scope
In this report, the Global Bispecific Antibodies Market has been segmented into the following categories:Bispecific Antibodies Market, by Indication:
- Cancer
- Inflammatory & Autoimmune Disorders
- Others
Bispecific Antibodies Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Bispecific Antibodies Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Bispecific Antibodies market report include:- Amgen Inc
- F. Hoffmann-La Roche Ltd
- Genentech Inc
- Akeso Inc
- Johnson & Johnson Private Limited
- Taisho Pharmaceutical Co Ltd
- Immunocore Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
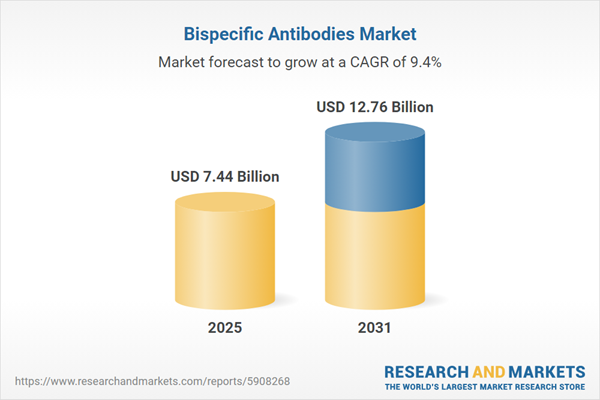
| Estimated Market Value ( USD | $ 7.44 Billion |
| Forecasted Market Value ( USD | $ 12.76 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |