Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the sector faces a substantial challenge due to the scarcity of available manufacturing slots and a shortage of highly skilled personnel, which limits the capacity of service providers to effectively handle the surging volume of projects. According to the Alliance for Regenerative Medicine, the sector displayed robust activity in 2025, with a 6 percent rise in the number of global developers and a 3 percent increase in clinical trials. This expansion of the development pipeline intensifies the pressure on existing manufacturing resources, underscoring the critical necessity for significant capacity expansion within the industry.
Market Drivers
The rapid expansion of cell and gene therapy clinical development pipelines serves as a primary catalyst for the Global Advanced Therapy Medicinal Products CDMO Market. As developers aggressively broaden their research initiatives, the immense volume of therapeutic candidates entering pre-clinical and clinical stages demands external manufacturing support to manage throughput. This demand is further amplified by strong sector financing, which supports the progression of these candidates through expensive development phases; for instance, the Alliance for Regenerative Medicine reported in October 2024 that the sector attracted USD 10.9 billion in investment during the first half of the year. Consequently, the industry depends heavily on contract partners, a reliance underscored by Citeline and the American Society of Gene & Cell Therapy's April 2024 report, which noted over 4,000 therapies in development globally.Simultaneously, the complexity of manufacturing advanced therapies is driving a strategic shift toward end-to-end integrated outsourcing models. Innovators encounter significant technical difficulties in scaling viral vector production and autologous cell processing, leading them to seek partners capable of providing a single-source solution from early-phase development through to commercialization. This comprehensive approach minimizes the logistical and regulatory risks associated with transferring technology between multiple vendors. In line with this trend, leading providers are investing heavily in integrated facilities; for example, Fujifilm Diosynth Biotechnologies committed an additional USD 1.2 billion in April 2024 to expand its large-scale cell culture facility, aiming to establish one of North America's largest end-to-end biopharmaceutical manufacturing sites.
Market Challenges
The scarcity of manufacturing slot capacity, coupled with a lack of highly skilled personnel, acts as a significant barrier to the growth of the Advanced Therapy Medicinal Products CDMO market. These constraints restrict the operational throughput of service providers, preventing them from fully absorbing the demand generated by biopharmaceutical innovators. Because these therapies require precise handling by experienced staff, Contract Development and Manufacturing Organizations are unable to rapidly scale their operations to meet client needs, which results in project backlogs and extended lead times for both clinical and commercial production.This bottleneck directly impacts market revenue growth by causing delays in project initiation and milestone completion. The gap between the high demand for development services and the limited supply of technical resources effectively creates a ceiling on market performance. According to the Alliance for Regenerative Medicine, there were over 1,900 active clinical trials globally in the regenerative medicine sector in 2024. This substantial volume of pipeline activity intensifies the competition for limited manufacturing slots, ensuring that capacity constraints remain a primary factor slowing the overall trajectory of the outsourcing sector.
Market Trends
The expansion of CDMO operations into Asia-Pacific hubs is reshaping the global supply chain as service providers build large-scale manufacturing clusters in the region to take advantage of skilled labor and government incentives. This geographical diversification addresses the industry's need for redundant capacity and lessens reliance on Western-centric production, particularly for commercial-scale biologic and advanced therapy manufacturing. Companies are aggressively constructing mega-plants in locations such as Singapore and South Korea to serve global clients with competitive timelines and costs. For instance, WuXi Biologics announced in July 2025 the development of a 30,000-square-meter modular drug product facility at Tuas Biomedical Park in Singapore, designed to enhance its global manufacturing network.concurrently, the market is shifting from transactional to strategic long-term partnerships, driven by the biopharmaceutical industry's need for guaranteed slot availability and supply chain security. Unlike traditional project-by-project outsourcing, these extended collaborations involve multi-year capacity reservations and dedicated production lines, effectively treating the CDMO as an extension of the client's internal network. This model provides financial stability for manufacturers while ensuring innovators have immediate access to resources during critical phases. According to Oxford Biomedica's interim results from September 2025, the company reported a contracted value of client orders totaling £149 million signed during the first half of the year, representing a 166 percent increase compared to the same period in 2024.
Key Players Profiled in the Advanced Therapy Medicinal Products CDMO Market
- Celonic AG
- Rentschler Biopharma SE
- Catalent, Inc.
- Lonza Group AG
- WuXi Advanced Therapies
- Minaris Regenerative Medicine, LLC
- Pluri Biotech Ltd.
- REPROCELL USA Inc.
- BioPhorum Operations Group
- Cellares Corporation
Report Scope
In this report, the Global Advanced Therapy Medicinal Products CDMO Market has been segmented into the following categories:Advanced Therapy Medicinal Products CDMO Market, by Product:
- Gene Therapy
- Cell Therapy
- Tissue Engineered
- Others
Advanced Therapy Medicinal Products CDMO Market, by Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
Advanced Therapy Medicinal Products CDMO Market, by Indication:
- Oncology
- Cardiology
- Central Nervous System
- Musculoskeletal
- Infectious disease
- Immunology & inflammation
- Others
Advanced Therapy Medicinal Products CDMO Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Advanced Therapy Medicinal Products CDMO Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Advanced Therapy Medicinal Products CDMO market report include:- Celonic AG
- Rentschler Biopharma SE
- Catalent, Inc.
- Lonza Group AG
- WuXi Advanced Therapies
- Minaris Regenerative Medicine, LLC
- Pluri Biotech Ltd.
- REPROCELL USA Inc.
- BioPhorum Operations Group
- Cellares Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
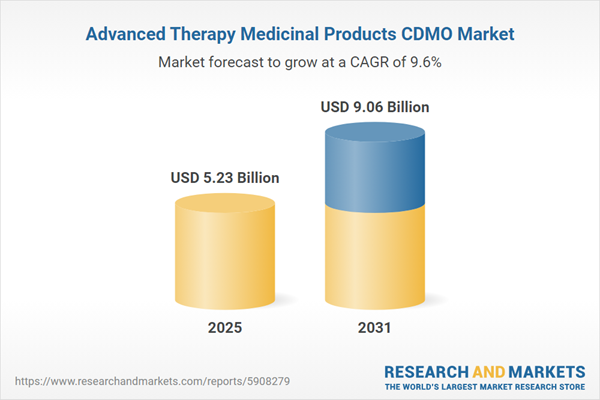
| Estimated Market Value ( USD | $ 5.23 Billion |
| Forecasted Market Value ( USD | $ 9.06 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |