Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The market is primarily shaped by enzyme replacement therapies (ERTs) such as Myozyme (alglucosidase alfa), Lumizyme, and the more recent Nexviazyme (avalglucosidase alfa), which offer improved glycogen clearance and clinical outcomes. Additionally, the approval of Pombiliti (cipaglucosidase alfa) and Opfolda (miglustat) in 2023 has expanded treatment options for patients, particularly those with late-onset Pompe disease (LOPD) who exhibit suboptimal responses to conventional ERTs.
Despite these advancements, market penetration is constrained by high treatment costs and limited newborn screening programs, leading to delayed diagnoses and disease progression. However, gene therapy and next-generation therapeutics are emerging as potential breakthroughs, with ongoing clinical trials aiming to provide more durable and cost-effective treatment alternatives.
Key Market Drivers
Increased Disease Awareness and Diagnosis
Pompe disease, often considered a rare or orphan disease, historically suffered from a lack of recognition and understanding. Pompe disease is a complex, multisystem genetic disorder with a prevalence of approximately 1 in 40,000 individuals in the United States. Increasing awareness of this rare condition is crucial, as early diagnosis and timely intervention can significantly improve patient outcomes and quality of life. Efforts to enhance disease recognition are being driven by medical institutions, patient advocacy groups, and biotech companies focused on expanding access to diagnostics and treatment.Newborn screening programs, physician education initiatives, and targeted awareness campaigns are playing a pivotal role in identifying patients earlier and facilitating access to advanced therapies. Patients faced delayed or misdiagnoses, leading to prolonged suffering and a dearth of effective treatments. Advocacy groups dedicated to Pompe disease have played a pivotal role in driving disease awareness. These organizations tirelessly work to raise public awareness, educate healthcare professionals, and provide support to affected individuals and their families.
By leveraging social media, organizing awareness campaigns, and collaborating with healthcare institutions, these groups have succeeded in putting Pompe disease on the radar. The evolution of diagnostic technologies is another catalyst for increased disease awareness. Cutting-edge tools such as genetic testing and biomarker identification have revolutionized the diagnostic process. Physicians can now identify Pompe disease more accurately and swiftly, enabling timely intervention and treatment initiation. This, in turn, has elevated the importance of early diagnosis in improving patient outcomes.
Key Market Challenges
Limited Disease Awareness and Late Diagnosis
One of the foremost challenges in the Pompe Disease Therapeutics Market is the limited awareness of the disease itself, leading to delayed or missed diagnoses. Pompe disease is considered a rare condition, making it less well-known among healthcare professionals and the general public. This lack of awareness often results in late-stage diagnoses, which can significantly impact treatment outcomes.Pompe disease's rarity, with an estimated incidence of 1 in 40,000 to 1 in 300,000 births, contributes to its obscurity. As healthcare professionals encounter rare diseases less frequently, the likelihood of prompt recognition and diagnosis diminishes. Pompe disease presents with symptoms that can overlap with those of other more common conditions, such as muscle weakness and respiratory problems. This lack of distinctiveness can lead to misdiagnoses or a failure to consider Pompe disease as a potential cause.
Key Market Trends
Personalized Medicine and Precision Therapies
One of the most significant trends in the Pompe Disease Therapeutics Market is the shift towards personalized medicine and precision therapies. Traditional treatment approaches often took a one-size-fits-all approach, but advancements in genetics and diagnostics have paved the way for tailored treatments that address the specific genetic mutations of individual patients. Advances in genetic research have provided insights into the precise genetic mutations that cause Pompe disease. This knowledge allows for the development of therapies that target the specific genetic defects in individual patients, maximizing treatment efficacy.Pharmaceutical companies are investing in innovative approaches, such as gene therapy and RNA-based therapies, that can be customized to target specific genetic mutations. These therapies hold the potential to correct the underlying genetic causes of Pompe disease. Advanced diagnostic tools, including genetic testing and biomarker identification, enable healthcare providers to identify the specific genetic mutations in Pompe disease patients. This information guides treatment decisions, ensuring that therapies are tailored to each patient's unique genetic profile.
Key Market Players
- Genzyme Corp
- Amicus Therapeutics Inc
- Valerion Therapeutics LLC
- Audentes Therapeutics Inc
- Actus Therapeutics Inc
- BioMarin Pharmaceutical Inc
- EpiVax inc
- Oxyrane Co Ltd.
- Sangamo BioSciences Inc
- Avrobio Inc
- Spark Therapeutics Inc
Report Scope:
In this report, the Global Pompe Disease Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Pompe Disease Therapeutics Market, By Treatment Type:
- Drugs
- Enzyme Replacement Therapy
- Physical Therapy
- Chaperone-Advanced Replacement Therapy
Pompe Disease Therapeutics Market, By Route of Administration:
- Oral
- Parenteral
Pompe Disease Therapeutics Market, By End User:
- Hospitals & Speciality Clinics
- Other
Pompe Disease Therapeutics Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Pompe Disease Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Genzyme Corp
- Amicus Therapeutics Inc
- Valerion Therapeutics LLC
- Audentes Therapeutics Inc
- Actus Therapeutics Inc
- BioMarin Pharmaceutical Inc
- EpiVax inc
- Oxyrane Co Ltd.
- Sangamo BioSciences Inc
- Avrobio Inc
- Spark Therapeutics Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
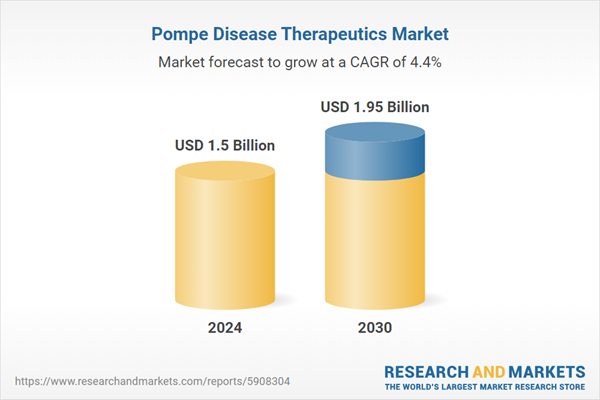
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 1.95 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |