Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these advancements, the market faces significant hurdles due to the immense heterogeneity of these conditions, which complicates the creation of standardized treatment protocols. According to the International Skeletal Dysplasia Society, in 2025, the field recognized 771 distinct skeletal disorders associated with hundreds of different gene mutations. This extensive variability creates a fragmented patient landscape, creating persistent challenges for industry stakeholders regarding the recruitment for clinical trials and the subsequent commercialization of broad-spectrum therapies.
Market Drivers
The introduction and approval of novel targeted pharmacotherapies is the primary force reshaping the Global Skeletal Dysplasia Market, transitioning the standard of care from palliative surgical management to disease-modifying interventions. This paradigm shift relies on the development of precision medicines, such as FGFR inhibitors and C-type natriuretic peptide analogs, which address the underlying molecular pathology of conditions like achondroplasia. These clinical advancements are rapidly broadening the therapeutic horizon, offering new hope for improving linear growth and proportionality in pediatric patients; for instance, Tyra Biosciences, Inc. announced in August 2025 that it had dosed the first pediatric patient with achondroplasia in its Phase 2 BEACH301 study, marking a critical milestone in next-generation oral therapy.Complementing this therapeutic innovation is the increasing investment in rare disease research and development, which provides the necessary capital to sustain complex, late-stage clinical programs. Pharmaceutical developers are leveraging robust financial positions to fund these high-cost trials, encouraged by the commercial potential of successful orphan drugs. This influx of capital is evident in the resource allocation of key industry players; BridgeBio Pharma, Inc. reported in January 2025 that it held $406 million to support its pipeline, including a fully enrolled Phase 3 dysplasia trial, while BioMarin Pharmaceutical Inc. illustrated the market's economic scale in February 2025 by reporting a record full-year 2024 revenue of $2.85 billion, underscoring the significant value generated by approved treatments in this sector.
Market Challenges
The immense heterogeneity of skeletal dysplasia conditions constitutes a severe impediment to market growth by creating a highly fragmented patient landscape. Because these disorders manifest through a vast array of unique genetic mutations, pharmaceutical developers face substantial difficulties in establishing standardized treatment protocols that are effective across a wide patient population. This fragmentation forces companies to target extremely narrow patient segments, which significantly complicates the clinical trial process; recruitment becomes inefficient and costly as finding a statistically significant cohort for a specific gene mutation often requires global search efforts, thereby delaying drug development timelines and increasing capital burn rates.Furthermore, this genetic diversity limits the commercial viability of potential therapies by restricting the addressable market for each individual treatment. According to the International Skeletal Dysplasia Society in 2025, the underlying genetic complexity of these conditions was attributed to 552 distinct causative genes. This high volume of genetic targets means that a therapeutic agent successful for one variant may be entirely ineffective for another, preventing the creation of broad-spectrum blockbusters and restricting revenue potential to niche indications, which continues to dampen overall market expansion due to the high risk associated with developing precision medicines for such small, distinct populations.
Market Trends
The Integration of Artificial Intelligence in Diagnostic Algorithms is revolutionizing the identification of skeletal dysplasias by automating radiographic analysis to distinguish between phenotypically similar conditions. This technological leap addresses the market's complexity, allowing clinicians to correlate bone abnormalities with genomic data and significantly reduce diagnostic timelines for rare variants. Illustrating this progress, the University of Bonn announced in January 2025 that it had secured a €1 million grant to advance "Bone2Gene," an AI-based software designed to classify over 700 forms of skeletal dysplasia from X-ray images, establishing a new standard for rapid clinical assessment.Simultaneously, the Emergence of Novel Gene-Editing Therapeutic Modalities is shifting the market from symptomatic management to curative, one-time interventions. Unlike conventional enzyme replacement therapies requiring lifelong administration, next-generation CRISPR-based platforms aim to permanently correct underlying mutations in conditions like Hypophosphatasia. This trend signifies a move towards durable biological solutions that restore natural bone mineralization; for instance, Be Biopharma released preclinical data in May 2025 for its CRISPR-Cas9 engineered B Cell Medicine, BE-102, demonstrating sustained enzyme delivery and highlighting the transformative potential of gene editing for rare bone disorders.
Key Players Profiled in the Skeletal Dysplasia Market
- Amgen Inc.
- Merck KGaA
- Regeneron Pharmaceuticals Inc.
- Alexion Pharmaceuticals Inc/MA
- Cipla Limited
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- Pfizer, Inc.
- AstraZeneca plc
Report Scope
In this report, the Global Skeletal Dysplasia Market has been segmented into the following categories:Skeletal Dysplasia Market, by Type:
- Achondroplasia
- Fibrodysplasia Ossificans Progressive
- Hypophosphatasia
- Multiple Osteochondromas
- X-linked Hypophosphatemia
Skeletal Dysplasia Market, by Treatment:
- Medication
- Surgery
Skeletal Dysplasia Market, by End User:
- Hospitals & Clinics
- Ambulatory Care Centers
- Others
Skeletal Dysplasia Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Skeletal Dysplasia Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Skeletal Dysplasia market report include:- Amgen Inc.
- Merck KGaA
- Regeneron Pharmaceuticals Inc
- Alexion Pharmaceuticals Inc/MA
- Cipla Limited
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- Pfizer, Inc.
- AstraZeneca PLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
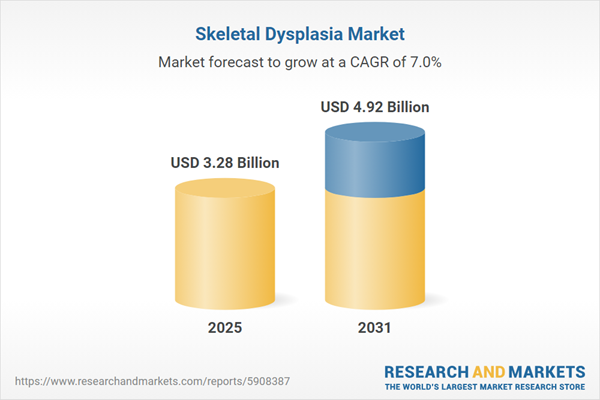
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 3.28 Billion |
| Forecasted Market Value ( USD | $ 4.92 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |