Syphilis Immunoassay Diagnostics: Introduction
Syphilis immunoassay is a type of diagnostic method used to check if a patient is affected with syphilis. Syphilis is a bacterial infection. It is a sexually transmitted disease which spreads through oral, vaginal, or anal sexual contact. It may even pass down from a pregnant woman to the fetus.The diagnosis is done via measuring certain antibody levels in the blood. It can be done through a blood test called rapid plasma regain (RPR) or through a blood or spinal fluid test called venereal disease research laboratory (VDRL) test.
Syphilis Immunoassay Diagnostics Market Analysis
Syphilis is the oldest known sexually transmitted disease, affecting a substantial portion of adults, falling in the age category of 15 to 49 years. Over the years, the incidence of Syphilis cases has increased by 32%. Therefore, there has been emphasis on diagnosing, preventing, and treating the disease. This has resulted in an increased syphilis immunoassay diagnostics market demand.Syphilis is caused by the gram-negative bacterium Treponema pallidum. Currently, most of the syphilis detection is investigated through T. pallidum IgG chemiluminescence immunoassay (CLIA), which is a qualitative assay performed to detect the antibodies against the bacteria in the body. Owing to the array of CLIA applications, Fapon Biotech has developed around 80 different CLIA tests. The CLIA Analyzer Shine i8000/9000 produces high outputs for rapid and reliable clinical diagnosis. It does not require any extensive maintenance either. Therefore, the syphilis immunoassay diagnostics market value is certain to rise with such technologies.
As syphilis can also be transmitted to newborns via mother, there has been focus on preventing developing methods to detect congenital syphilis in children. The researchers from UTHealth Houston are developing a medical diagnostic test for the detection of congenital syphilis to facilitate early treatment. This method can turn out to be promising in the future as 60% of untreated syphilis infected infants suffer from neurodevelopmental issues.
Syphilis Immunoassay Diagnostics Market Segmentation
Syphilis Immunoassay Diagnostics Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Analyzers
- Kits
- Reagents
Market Breakup by Technology
- CLIA
- ELISA
- Others
Market Breakup by End User
- Hospitals
- Diagnostic Labs
- Blood Banks
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
Syphilis Immunoassay Diagnostics Market Overview
The United States is expected to lead the syphilis immunoassay diagnostics market share. As sexually transmitted diseases continue to rise in the region, the government has been setting several awareness campaigns to spread awareness and prevent the illness. ‘Girl, Get Tested' is a notable public awareness campaign promoting syphilis screening in cisgender women. In addition, the United States also has prominent healthcare companies which continue to bring newer technologies.The Asia Pacific region, especially Japan, is estimated to observe fastest growth, owing to the increasing prevalence of developing a well-equipped infrastructure for research and development. The syphilis immunoassay diagnostics market growth can be accredited to several awareness initiatives. For example, in March 2023, the Australian government initiated ‘Let your partners know' syphilis awareness campaign envisioned to encourage contact tracing for people who have tested positive to syphilis. The aim is to ultimately increase syphilis testing and treatment.
Syphilis Immunoassay Diagnostics Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- BioRad Laboratories Inc
- Danaher Corporation
- BECTON DICKINSON & COMPANY
- F. Hoffmann-La Roche AG
- Siemens Healthineers AG
- Diasorin S.P.A
- Abbott
- Fujirebio
- BioMerieux SA
- Shenzhen New Industries Biomedical Engineering Co. Ltd
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- BioRad Laboratories Inc
- Danaher Corporation
- BECTON DICKINSON & COMPANY
- F. Hoffmann-La Roche AG
- Siemens Healthineers AG
- Diasorin S.P.A
- Abbott
- Fujirebio
- BioMerieux SA
- Shenzhen New Industries Biomedical Engineering Co. Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
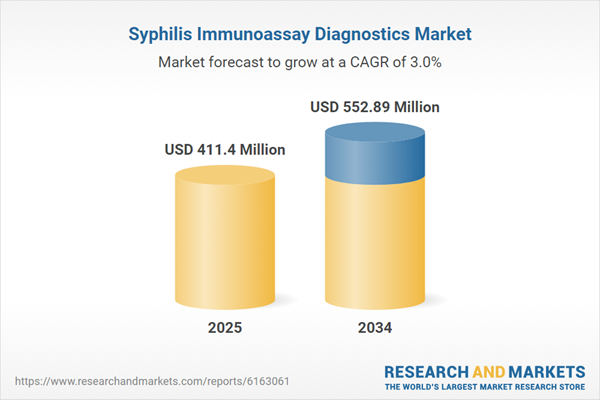
| Estimated Market Value ( USD | $ 411.4 Million |
| Forecasted Market Value ( USD | $ 552.89 Million |
| Compound Annual Growth Rate | 3.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |