Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these favorable conditions, the market confronts substantial hurdles due to the clinical heterogeneity of the disease, which makes accurate diagnosis and targeted therapy selection difficult. This complexity often results in poor treatment adherence and significant management challenges for patients. For example, the MDS Alliance reported in 2024 that 45% of patients were unaware of their specific MDS subtype, a lack of diagnostic clarity that limits the effective use of advanced therapies and restricts the potential growth of the specialized treatment sector.
Market Drivers
A strong pipeline of innovative therapeutic agents is fundamentally transforming the Global MDS Treatment Market, moving the standard of care from merely supportive measures to disease-modifying interventions. Developers are prioritizing telomerase inhibitors and other new mechanisms to overcome the shortcomings of traditional erythropoiesis-stimulating agents, especially for patients dependent on transfusions. This innovation is vital given the disease's poor prognosis; the American Society of Clinical Oncology noted in March 2024 that the 5-year relative survival rate is around 37%. Regulatory successes underscore this progress, such as Geron Corporation’s June 2024 announcement regarding the FDA approval of Rytelo, where the IMerge Phase 3 trial showed a 39.8% red blood cell transfusion independence rate for at least eight weeks with imetelstat.In parallel, the rise of targeted and immunomodulatory therapies is fueling market growth by allowing for more precise treatment of specific disease subtypes, particularly in first-line settings. These therapies, which address specific biological pathways and maturation defects, are seeing increased adoption and generating significant commercial value over older, non-specific treatments. This shift is reflected in the financial results of major players; Bristol Myers Squibb reported in October 2024 that worldwide revenues for Reblozyl jumped 80% to $447 million, driven by robust demand in first-line MDS treatment, indicating a market consolidation around high-value pharmacotherapies that deliver better outcomes than traditional care.
Market Challenges
The clinical heterogeneity inherent in Myelodysplastic Syndromes poses a major constraint on the growth of the global treatment market. Since the disease manifests with diverse biological features and progression speeds, physicians often struggle to pinpoint accurate diagnoses and determine the best therapeutic courses. This variability frequently results in delayed treatment or the choice of suboptimal management plans, thereby dampening the utilization of targeted pharmacotherapies and creating barriers for manufacturers attempting to widely distribute drugs tailored to specific biological subtypes.Such diagnostic complexity fragments the patient base and curbs the revenue potential for advanced treatments. Without precise classification, patients are frequently assigned to general supportive care rather than receiving high-value active therapies. Although the American Cancer Society estimates that 13,000 new MDS cases will be diagnosed in the United States in 2025, the absence of a standardized disease presentation prevents the market from fully addressing this demand, as diagnostic uncertainty limits the consistent prescription of premium medical interventions.
Market Trends
The shift toward Oral Hypomethylating Agents is significantly reshaping the treatment landscape by moving care from intravenous infusions to home-based regimens. This trend mitigates the logistical challenges of chronic parenteral therapies, providing a more convenient option that aids adherence and decreases the frequency of clinic visits. Real-world data supports the safety of this transition; an abstract from the American Society of Hematology in November 2025 revealed that patients on oral decitabine-cedazuridine had a notably lower incidence of febrile neutropenia during the first two cycles - 17% compared to 55% for those on parenteral agents - highlighting the potential for fewer hospitalizations.At the same time, the market is characterized by a surge in Combination Therapy Clinical Trials, where pharmaceutical companies are testing hypomethylating agents paired with novel mechanisms like BCL-2 inhibition to surpass the efficacy limits of monotherapy. While this research aims to enhance survival in higher-risk groups through synergistic pathways, achieving statistically significant clinical results remains difficult. For instance, AbbVie announced in June 2025 that the pivotal Phase 3 VERONA trial, testing venetoclax combined with azacitidine in newly diagnosed higher-risk patients, failed to meet its primary overall survival endpoint, showing a hazard ratio of 0.908.
Key Players Profiled in the Myelodysplastic Syndrome (MDS) Treatment Market
- AbbVie Inc.
- Accord Healthcare Limited
- Bristol-Myers Squibb Company
- Jazz Pharmaceuticals Inc.
- Novartis AG
- Lupin Limited
- Otsuka America Pharmaceutical Inc.
- Takeda Pharmaceutical Company Limited
- Astex Therapeutics Limited
- Pfizer Inc.
Report Scope
In this report, the Global Myelodysplastic Syndrome (MDS) Treatment Market has been segmented into the following categories:Myelodysplastic Syndrome (MDS) Treatment Market, by Treatment Type:
- Stem Cell Transplant
- Immune Treatments
- Chemotherapy
- Immunomodulatory Drugs
- Anti-anemics
- Others
Myelodysplastic Syndrome (MDS) Treatment Market, by End User:
- Hospitals & Clinics
- Ambulatory Care Centers
- Others
Myelodysplastic Syndrome (MDS) Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Myelodysplastic Syndrome (MDS) Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Myelodysplastic Syndrome (MDS) Treatment market report include:- AbbVie Inc.
- Accord Healthcare Limited
- Bristol-Myers Squibb Company
- Jazz Pharmaceuticals Inc.
- Novartis AG
- Lupin Limited
- Otsuka America Pharmaceutical Inc.
- Takeda Pharmaceutical Company Limited
- Astex Therapeutics Limited
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
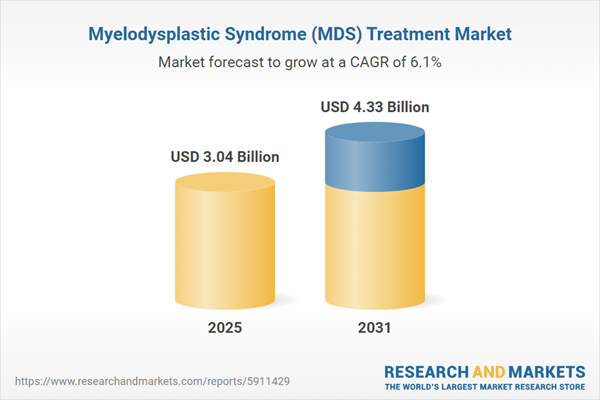
| Estimated Market Value ( USD | $ 3.04 Billion |
| Forecasted Market Value ( USD | $ 4.33 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |