Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Conversely, a major hurdle limiting extensive market growth is the self-limiting nature of the illness, which diminishes the commercial motivation for pharmaceutical firms to engineer expensive, targeted antiviral treatments. Consequently, the industry relies heavily on low-margin generic drugs, which constrains total revenue potential despite the large volume of cases. To illustrate the significant burden of the disease, data from the Taiwan Centers for Disease Control indicates that in 2024, weekly outpatient and emergency visits for enterovirus infections reached a peak of over 22,000 cases during the height of the epidemic season.
Market Drivers
The increasing global prevalence of pediatric herpangina serves as a major catalyst for market expansion, ensuring the continuous use of medications for symptomatic relief. As enteroviral transmission rates climb in the post-pandemic landscape, healthcare professionals are observing a marked increase in young patients needing clinical care for fever and painful oropharyngeal ulcers. This epidemiological surge leads directly to greater sales of analgesics, anti-inflammatory agents, and topical anesthetics, which function as the primary treatment methods lacking specific antivirals. Demonstrating this trend, the National Economic and Social Development Council of Thailand reported in its 'Second Quarter 2025 Economic Report' in September 2025 that cases of hand, foot, and mouth disease - the main clinical manifestation of these enteroviruses - rose by 65.7% year-on-year during that quarter, compelling manufacturers to sustain high production levels.Furthermore, government efforts to control infectious diseases boost market activity through strict surveillance and the accelerated approval of preventive measures. Health authorities are enacting stronger monitoring protocols and backing the creation of vaccines to reduce the impact of severe strains like EV71, which validates prescribed therapies and promotes early medical intervention. For example, the Vietnam Ministry of Health, in a February 2025 'Joint Statement on Hand-Foot-Mouth Disease', emphasized the critical need to approve the nation's first domestic vaccine following more than 76,000 recorded cases the prior year. Similarly, the Korea Disease Control and Prevention Agency’s '2024 Annual Report on Notified Infectious Diseases', released in July 2025, showed a 54.5% rise in total infectious cases, highlighting the urgent requirement for robust disease management strategies.
Market Challenges
The self-limiting characteristic of herpangina represents a significant barrier to the financial expansion of the global treatment market. Since this viral infection usually clears up on its own within a week without leaving lasting effects, there is little clinical urgency to prescribe potent, expensive antiviral therapies. This brief duration markedly reduces the commercial feasibility of creating disease-specific medications, as pharmaceutical manufacturers view the return on investment for such niche products as inadequate. As a result, the market depends heavily on low-margin over-the-counter analgesics and antipyretics, limiting the total revenue potential for industry players despite frequent outbreaks.This dynamic fosters a market landscape defined by high case numbers but disproportionately low financial value. While the widespread nature of the ailment guarantees consistent demand for supportive care, it does not lead to the uptake of premium therapeutic options. Highlighting this gap, the National Institute of Infectious Diseases reported that in 2025, weekly herpangina cases in Japan peaked at roughly 4.0 per sentinel site during the summer epidemic season. This data underscores the existence of a large patient population that, due to the temporary nature of the condition, generates only minimal high-value revenue for the pharmaceutical industry.
Market Trends
The integration of Rapid Molecular Diagnostic Technologies is revolutionizing the clinical approach to herpangina by facilitating exact pathogen identification. Medical providers are progressively moving away from reliance on symptoms alone to employing advanced multiplex PCR assays, which are essential for differentiating herpangina-causing Coxsackieviruses from other dangerous enteroviral strains with similar symptoms. This technological advancement improves patient triage and curtails the unnecessary use of antibiotics for viral infections. Emphasizing the need for such precise surveillance, the American Society for Microbiology noted in its June 2025 report, 'Enhanced genomic surveillance of enteroviruses', that detailed molecular screening identified EV-D68 in 72.6% of enterovirus-positive samples, highlighting the vital function of advanced diagnostics in accurate disease tracking.Simultaneously, a specific research emphasis on Broad-Spectrum Anti-Enteroviral Agents is developing to tackle the absence of targeted curative treatments. Acknowledging the constraints of purely symptomatic care, pharmaceutical developers are vigorously investigating new therapeutic categories, including capsid inhibitors and protease inhibitors, intended to combat a broad spectrum of enterovirus serotypes. This strategic shift steers the market away from low-cost analgesics toward high-value disease-modifying medications, specifically to avert severe complications in at-risk pediatric groups. Confirming the significant economic promise of this direction, GeneOnline projected in its December 2025 article, 'Enterovirus Hits 6-Year Peak', that the non-polio enterovirus therapeutics sector will surpass $10 billion by 2030, fueled by the pressing demand for effective treatments.
Key Players Profiled in the Herpangina Treatment Market
- Gilead Sciences Inc.
- Johnson and Johnsons
- Merck & Co. Inc.
- Pfizer Inc.
- AbbVie Inc.
- Vertex Pharmaceuticals Incorporated
- Sanofi S.A
- Novartis AG
- Mitsubishi Tanabe Pharma Corporation
- F. Hoffmann-La Roche Ltd.
Report Scope
In this report, the Global Herpangina Treatment Market has been segmented into the following categories:Herpangina Treatment Market, by Treatment:
- Topical Anesthetics
- Ibuprofen or Acetaminophen
- Other Treatments
Herpangina Treatment Market, by Virus:
- Coxsackie Virus A
- Coxsackie Virus B
- Enterovirus 71
- Echovirus
Herpangina Treatment Market, by End User:
- Hospitals
- Homecare
- Specialty Clinics
- Others
Herpangina Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Herpangina Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Herpangina Treatment market report include:- Gilead Sciences Inc
- Johnson and Johnsons
- Merck & Co. Inc
- Pfizer Inc.
- AbbVie Inc.
- Vertex Pharmaceuticals Incorporated
- Sanofi S.A
- Novartis AG
- Mitsubishi Tanabe Pharma Corporation
- F. Hoffmann-La Roche Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
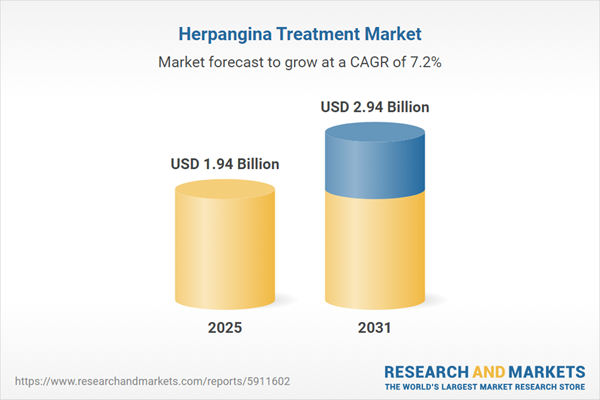
| Estimated Market Value ( USD | $ 1.94 Billion |
| Forecasted Market Value ( USD | $ 2.94 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |