Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these drivers, the market encounters significant obstacles due to the high costs and intricate nature of manufacturing, which limits availability in resource-constrained regions. These complex production demands frequently result in supply bottlenecks and elevated prices that hinder universal access. To illustrate the current scale of deployment, the World Health Organization reported in 2025 that the pneumococcal conjugate vaccine had been introduced in 163 Member States by late 2024, achieving a global third-dose coverage of 67%. This statistic underscores both the widespread distribution of these biologics and the persistent gaps remaining in global immunization coverage.
Market Drivers
Strategic initiatives by global health organizations to enhance vaccine availability are fundamentally reshaping the market landscape. These entities collaborate with governments to introduce conjugate vaccines into national programs, particularly in high-burden areas such as the African meningitis belt. By securing supply chains and subsidizing procurement costs, these initiatives ensure that life-saving formulations reach vulnerable populations that were previously underserved. A prime example of this expansion occurred when a major African nation integrated a novel pentavalent meningococcal conjugate vaccine into its public health strategy. According to Gavi, the Vaccine Alliance, in an April 2024 press release regarding Nigeria's introduction of the Men5CV vaccine, the alliance supported an emergency campaign targeting 1 million people to halt an outbreak, demonstrating the scale of these coordinated efforts.Concurrently, a strong clinical pipeline and the launch of novel multivalent vaccines are driving market growth and broadening the scope of protection. Manufacturers are increasingly shifting focus from pediatric-only indications to comprehensive adult strategies, developing formulations that cover a wider array of serotypes to mitigate antimicrobial resistance.
This innovation aims to provide broader coverage against evolving bacterial strains, offering healthcare providers more potent tools to prevent invasive disease. Highlighting this advancement, Merck & Co., Inc. announced in June 2024 that the FDA approved CAPVAXIVE, the first 21-valent pneumococcal conjugate vaccine specifically designed for adults. Such commercial milestones translate into significant financial value; for instance, Pfizer Inc. reported that its Prevnar franchise generated $1.8 billion in global revenues during the third quarter of 2024 alone.
Market Challenges
The primary obstacle hampering the growth of the Global Conjugate Vaccine Market is the substantial cost and technical complexity associated with the manufacturing process. Producing these biologics requires intricate chemical conjugation procedures to link bacterial polysaccharides with protein carriers, a step that demands highly specialized facilities and advanced technical expertise. These rigorous production standards necessitate significant capital investment in infrastructure and quality control systems, creating high barriers to entry for new manufacturers. Consequently, the market remains concentrated among a limited number of major pharmaceutical players, which restricts competition and keeps unit prices elevated, thereby limiting procurement volumes in cost-sensitive markets.This concentration of manufacturing capacity results in supply chain vulnerabilities and unequal access, particularly in developing regions where local production capabilities are absent. The inability to manufacture these complex formulations locally forces reliance on expensive imports, which often leads to supply shortages and slower market penetration. Illustrating this disparity, Gavi, the Vaccine Alliance reported in 2024 that the African continent produced only approximately 0.1% of the global vaccine supply, leaving the region almost entirely dependent on imported doses. This lack of distributed manufacturing capability hampers the market’s potential to expand efficiently into high-demand, resource-constrained geographies.
Market Trends
Advancements in synthetic conjugate vaccine manufacturing are fundamentally altering production paradigms by moving beyond traditional chemical conjugation methods. This trend is characterized by the adoption of cell-free protein synthesis and site-specific linkage technologies, which circumvent the technical limitations of conventional manufacturing.Unlike standard processes that struggle with carrier suppression and steric hindrance when adding multiple serotypes, synthetic platforms enable the precise assembly of high-valency formulations without compromising immunogenicity. The commercial viability of this technological shift is evident in the substantial capital flowing into companies pioneering these methods; for example, Vaxcyte, Inc. announced in September 2024 that it raised significant funds through a $1.3 billion public offering to advance its synthetic vaccine pipeline aimed at producing broader-spectrum protection.
Simultaneously, the emergence of therapeutic conjugate vaccines for non-communicable diseases represents a divergence from the market’s traditional infectious disease focus. Researchers and startups are increasingly leveraging conjugate technology to treat substance use disorders by engineering formulations where drug haptens, such as fentanyl or heroin derivatives, are coupled to immunogenic carrier proteins. These vaccines induce the production of antibodies that sequester the target drug in the bloodstream, preventing it from crossing the blood-brain barrier and thereby neutralizing its psychoactive effects. This application expands the addressable market into addiction medicine, attracting new streams of investment. As reported by The Dallas Morning News in September 2024, the biopharmaceutical startup Ovax had raised over $10 million by June 2024 to progress a novel fentanyl conjugate vaccine into human clinical trials.
Key Players Profiled in the Conjugate Vaccine Market
- Sanofi S.A
- Pfizer Inc.
- Merck & Co. Inc.
- GlaxoSmithKline PLC
- Bharat Biotech International Limited
- Serum Institute of India Pvt. Ltd.
- Biological E. Limited
- Bavarian Nordic A/S
- CSL Limited
- Novartis AG
Report Scope
In this report, the Global Conjugate Vaccine Market has been segmented into the following categories:Conjugate Vaccine Market, by Product Type:
- Monovalent Conjugate Vaccines
- Multivalent Conjugate Vaccine
Conjugate Vaccine Market, by Disease Indication:
- Pneumococcal
- Influenza
- Meningococcal
- Typhoid
Conjugate Vaccine Market, by End-User:
- Hospitals & Clinics
- Ambulatory care Centers
- Others
Conjugate Vaccine Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Conjugate Vaccine Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Conjugate Vaccine market report include:- Sanofi S.A
- Pfizer Inc.
- Merck & Co. Inc.
- GlaxoSmithKline PLC
- Bharat Biotech International Limited
- Serum Institute of India Pvt. Ltd
- Biological E. Limited
- Bavarian Nordic A/S
- CSL Limited
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
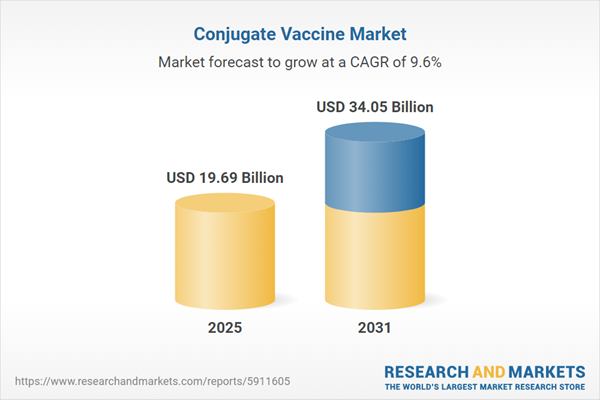
| Estimated Market Value ( USD | $ 19.69 Billion |
| Forecasted Market Value ( USD | $ 34.05 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |