The need for IgA nephropathy disease treatment is estimated to grow over the coming years on account of the increasing prevalence of kidney diseases. Research is being conducted to develop new and effective treatment methods and continue to offer growth opportunities for the market players during the forecast period.
Moreover, clearances and approval of new drugs as well as major campaigns establishments for increasing awareness among people regarding IgA Nephropathy disease and health checkups will have a positive impact on the market growth. For instance, the US Food and Drug Administration, in September 2022, provided clearance to the Investigational New Drug (IND) Application of Eldon Pharmaceutical for assessment of tegoprubart to treat IgA Nephropathy.
Additionally, in May 2022, Calliditas launched its educational program on IgA Nephropathy called 'IgAN Connect' which will provide necessary tools and sources to people who have either recently been diagnosed with IgA Nephropathy or are living for many years. Such drug approval and clearances coupled with education programs relating to IgA Nephropathy present a positive outlook for the IgA Nephropathy market over the coming years
IGA nephropathy disease treatment market drivers
Growing prevalence of chronic kidney disease is driving the market growth globally.The incidences of chronic kidney diseases are increasing the demand for IgA nephropathy which is boosting the market growth. The development of new pathogenic mechanisms and a strong pipeline of innovative prospective therapeutics are anticipated to both play a significant role in the growth of the IgA Nephropathy market.
In addition, sedentary lifestyles, excessive smoking, increased alcohol consumption, and an increase in obesity all contribute to the disease's rising occurrence. For instance, as per the report of Center for Disease Control and Prevention, published in 2023, the people suffering from CKD in the United States with an age range between 18 to 44 was 6.30% of the overall population of that age range, for people aged between 45-64 was 12.30% and that for 65+ people it was 33.70%, hence the IgA nephropathy disease treatment market is anticipated to experience considerable expansion throughout the projected period.
IGA nephropathy disease treatment market geographical outlook
North America will continue to hold a remarkable share of the market during the forecast period.Geographically, the IGA nephropathy disease treatment market has been segmented into North America, South America, Europe, the Middle East, Africa, and the Asia-Pacific.
During the forecast period, the North American region is expected to grow at a constant rate and will constitute a considerable market share fueled by the growing chronic disease prevalence in major regional economies. According to the US National Institute of Diabetes and Digestive and Kidney Disease, Department of Health and Human Services, Chronic kidney disease (CKD) affects more than one in seven US Adults.
And according to the Center For Disease Control and Prevention, nearly 14% of the country's total population or 35.5 million Americans suffer from chronic kidney disease. Moreover, the same source also specified that by gender, women are more prone to be affected by chronic kidney disease as compared to men.
Reasons for buying this report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape up future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decision to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive IntelligenceReport Coverage:
- Historical data & forecasts from 2022 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, Customer Behaviour, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others)
The IGA Nephropathy Disease Treatment Market is analyzed into the following segments:
By Test
- Blood Test
- Urine Test
- Kidney Test
- Iothalamate Clearence Test
- Others
By Treatment Type
- Corticosteroids
- Immunosuppressive Drugs
- Ace Inhibitors and Arbs
- Diet Change
- Therapy
By Geography
- North America
- South America
- Europe
- Middle East and Africa
- Asia-Pacific
Table of Contents
Companies Mentioned
- TRAVERE THERAPEUTICS INC.
- CALLIDITAS THERAPEUTICS AB
- Omeros
- NOVARTIS PHARMACEUTICALS
- CHINOOK THERAPEUTICS INC
- VERA THERAPEUTICS, INC
- Merck KGAA
- REATA PHARMACEUTICALS INC.
- IONIS PHARMACEUTICALS
- Stada Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 132 |
| Published | December 2024 |
| Forecast Period | 2025 - 2030 |
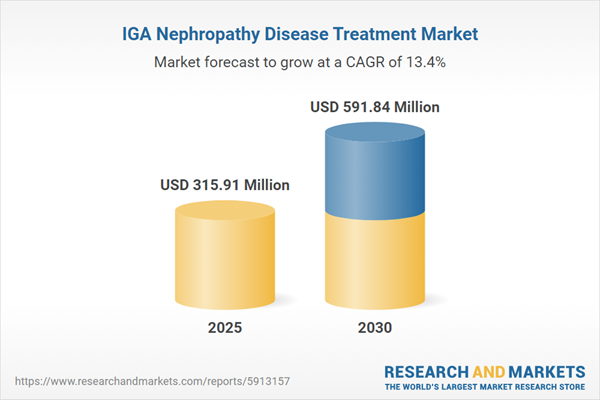
| Estimated Market Value ( USD | $ 315.91 Million |
| Forecasted Market Value ( USD | $ 591.84 Million |
| Compound Annual Growth Rate | 13.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |