Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces a significant obstacle in the form of complex reimbursement structures for new device technologies, which often limit patient access and adoption within budget-conscious healthcare systems. Highlighting the magnitude of this issue, the American Urological Association reported in 2024 that the prevalence of Benign Prostatic Hyperplasia in men aged 65 and older ranged between 29% and 35%. This high prevalence reinforces the critical and increasing need for accessible therapeutic options within the sector.
Market Drivers
Technological innovations in robotic-assisted systems and laser ablation are transforming the market by emphasizing precision and faster recovery times. These advancements are rapidly replacing traditional transurethral resections as both clinicians and patients favor automated, tissue-sparing methods that reduce sexual side effects and hospital stays. The financial success of emerging market leaders reflects this shift; for instance, PROCEPT BioRobotics reported a 66% year-over-year revenue increase to $58.4 million in its Third Quarter 2024 Financial Results, driven largely by the strong adoption of its Aquablation robotic systems.Additionally, the growing global geriatric population serves as a major demographic driver for long-term market growth, due to the link between aging and prostate enlargement. As life expectancy rises, the number of men developing lower urinary tract symptoms increases, creating a need for scalable medical solutions. The United Nations Population Fund's '2024 State of World Population Report' noted that those aged 65 and older constituted 10.3% of the global population in 2024, indicating a major shift in patient density. This demographic trend creates commercial opportunities, as seen in Boston Scientific's February 2025 report, where the Urology segment saw a 12.0% rise in full-year 2024 net sales, confirming rising demand among the elderly.
Market Challenges
The growth of the Global Benign Prostatic Hyperplasia Treatment Devices Market is significantly hindered by a complicated reimbursement environment for innovative technologies, which creates financial hurdles for healthcare providers. New devices frequently launch without established billing codes or favorable insurance coverage, compelling medical facilities to either absorb the costs or navigate unpredictable payment models. This financial uncertainty deters hospitals and urology practices from purchasing expensive equipment, leading them to prefer established, fully reimbursed procedures over newer, potentially more beneficial alternatives.As a result, urologists experience shrinking profit margins that further limit their capacity to incorporate emerging therapies. According to the American Urological Association in 2024, the Centers for Medicare & Medicaid Services finalized a payment rule reducing the Medicare conversion factor by approximately 2.83 percent for the following year. This cut in reimbursement rates intensifies financial strain on providers, directly restricting their ability to adopt novel BPH treatment devices and impeding overall market growth in cost-sensitive healthcare settings.
Market Trends
The introduction of temporary implantable nitinol devices marks a significant shift toward non-permanent, mechanical reshaping of the prostatic urethra, distinguishing these tools from permanent stents or ablative techniques. Devices such as the iTind system are engineered to remodel the bladder neck while preserving sexual function by avoiding thermal injury or permanent implants, thereby addressing a key quality-of-life concern for patients. This innovative method is gaining commercial momentum as manufacturers expand into key regions; for example, Olympus Corporation reported in August 2024 that its North American revenue rose by 32% year-over-year in the first quarter of fiscal year 2025, a result partly linked to the strategic market development of its iTind device.Simultaneously, the broad commercialization of water vapor thermal therapy is setting a new benchmark for office-based procedures by using convective steam energy to treat hyperplasia effectively. Unlike robotic or laser enucleation, this modality delivers sterile water vapor into prostate tissue, rapidly dispersing thermal energy to cause cell death without requiring extensive surgical infrastructure. The technology's rapid adoption is driven by its ability to provide lasting symptom relief with short procedure times, contributing to growth for major device companies; Boston Scientific's 'Third Quarter 2024 Financial Results' in October 2024 showed a 10.3% increase in Urology net sales, bolstered by double-digit growth in its prostate health franchise, including the Rezūm Water Vapor Therapy system.
Key Players Profiled in the Benign Prostatic Hyperplasia Treatment Devices Market
- Boston Scientific Corporation
- Olympus America
- Endo Pharmaceuticals Inc.
- biolitec AG
- Medifocus Inc.
- Cook Medical
- Teleflex Incorporated
- Urotronic
- Coloplast Corp
- Richard Wolf GmbH
- PROCEPT BioRobotics Corporation
Report Scope
In this report, the Global Benign Prostatic Hyperplasia Treatment Devices Market has been segmented into the following categories:Benign Prostatic Hyperplasia Treatment Devices Market, by Treatment:
- Minimal Invasive Surgery
- Invasive Surgery
Benign Prostatic Hyperplasia Treatment Devices Market, by Procedure:
- Transurethral Resection of the Prostate
- Prostate Laser Surgery
- Transurethral Microwave Thermotherapy
- Transurethral Needle Ablation of the Prostate
- Prostatic Urethral Lift
- Water Vapor Therapy
- Others
Benign Prostatic Hyperplasia Treatment Devices Market, by Product:
- Resectoscopes
- Urology Laser
- Radiofrequency Ablation
- Electrodes
- Catheters
- Prostatic Stents
- Implants
- Others
Benign Prostatic Hyperplasia Treatment Devices Market, by End use:
- Hospitals & Clinics
- Research and Manufacturing
- others
Benign Prostatic Hyperplasia Treatment Devices Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Benign Prostatic Hyperplasia Treatment Devices Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Benign Prostatic Hyperplasia Treatment Devices market report include:- Boston Scientific Corporation
- Olympus America
- Endo Pharmaceuticals Inc.
- biolitec AG
- Medifocus Inc.
- Cook Medical
- Teleflex Incorporated
- Urotronic
- Coloplast Corp
- Richard Wolf GmbH
- PROCEPT BioRobotics Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
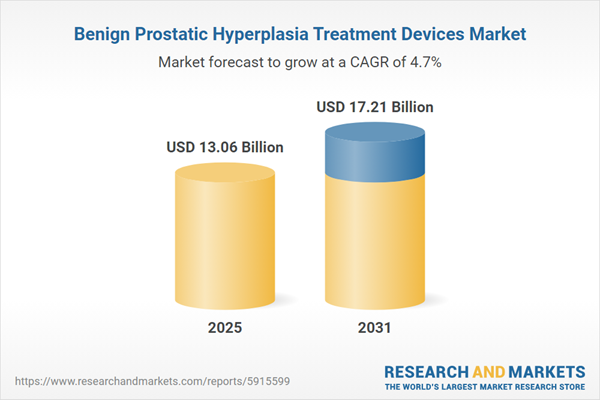
| Estimated Market Value ( USD | $ 13.06 Billion |
| Forecasted Market Value ( USD | $ 17.21 Billion |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |