Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite this growth trajectory, the market faces a significant obstacle concerning the overdiagnosis of indolent thyroid nodules, a practice that frequently results in unnecessary surgeries and overtreatment. In response, regulatory authorities have revised screening guidelines to recommend more conservative management for small, low-risk nodules. These changes in clinical practice create uncertainty regarding reimbursement and decrease the number of diagnostic procedures conducted for minor cases, which subsequently hinders broader market expansion.
Market Drivers
The escalating global incidence of thyroid cancer serves as a major driver for the diagnostics market, creating a necessity for robust screening protocols to accommodate increasing patient numbers. This surge is especially noticeable among younger populations, forcing healthcare systems to enhance their diagnostic capacities to facilitate earlier intervention. According to the World Health Organization's International Agency for Research on Cancer study titled 'Thyroid cancer in adolescents and young adults' from November 2025, there were over 237,000 new cases globally within this demographic in 2022. This increasing burden is also reflected in national statistics; for example, Cancer Research UK reported in September 2025 that approximately 4,000 new thyroid cancer cases are diagnosed annually in the region, emphasizing the ongoing requirement for effective detection methods across various populations.In parallel, the rising integration of molecular diagnostics and genomic profiling is revolutionizing clinical workflows by resolving the difficulty of managing indeterminate thyroid nodules. Advanced genomic classifiers enable physicians to differentiate between benign and malignant nodules with improved precision, thereby lowering the rate of unnecessary diagnostic surgeries and ensuring better resource utilization. This movement toward precision medicine is demonstrated by the significant adoption of specialized testing products. In its 'Fourth Quarter and Full Year 2024 Financial Results' released in February 2025, Veracyte announced that its Afirma genomic test volume increased by 12% to exceed 60,000 tests for the full year 2024. Such strong adoption figures suggest that molecular testing is quickly becoming a standard of care, boosting market revenue through the use of higher-value diagnostic procedures.
Market Challenges
The principal obstacle restricting the thyroid cancer diagnostics market is the clinical shift aimed at mitigating the overdiagnosis of indolent thyroid nodules. Although the detection of minute, low-risk nodules historically triggered aggressive diagnostic evaluations, current practice standards increasingly prioritize active surveillance instead of immediate fine needle aspiration biopsy or molecular testing for small lesions. This move toward conservative management directly lowers the frequency of diagnostic interventions, thereby reducing the total volume of tests conducted and constraining revenue streams for diagnostic providers.This decline in procedural volume creates a challenging landscape for market growth, as healthcare systems implement stricter reimbursement criteria to eliminate unnecessary expenses related to low-risk cases. According to estimates from the Canadian Cancer Society, approximately 6,900 individuals in Canada will be diagnosed with thyroid cancer in 2025. Although this incidence rate adds to the potential patient pool, the growing selectivity in clinical guidelines implies that a smaller proportion of patients with thyroid nodules will receive comprehensive diagnostic profiling. As a result, companies encounter limited utilization of premium diagnostic tools, since providers are restricting these advanced modalities to high-risk malignancies rather than using them for the routine screening of minor nodules.
Market Trends
The incorporation of Artificial Intelligence and Machine Learning into imaging is fundamentally transforming thyroid cancer detection by improving ultrasound accuracy and optimizing clinical operations. AI algorithms are increasingly being utilized to assess sonographic characteristics, assisting clinicians in distinguishing between benign and malignant nodules with greater precision than traditional visual analysis provides. This technological progress addresses the inconsistency in operator performance and notably boosts practice efficiency. For instance, RamSoft's September 2025 press release, 'RamSoft Partners with Koios Medical,' noted that integrating Koios DS AI software into radiology platforms allows clinics to reduce interpretation time by 49% while enhancing patient throughput, enabling providers to handle growing patient volumes more effectively without sacrificing diagnostic confidence.At the same time, the rise of Companion Diagnostics for Targeted Therapies is fueling the growth of rapid next-generation sequencing (NGS) applications for advanced thyroid malignancies. As new therapies that target specific mutations such as RET and BRAF become standard practice, there is an urgent requirement for rapid and extensive genomic profiling to inform treatment choices immediately following diagnosis.
This trend extends beyond basic nodule classification to focus on detecting actionable alterations in aggressive cancers to refine therapeutic strategies. A report in CAP TODAY from August 2025, titled 'Introduction of a new FDA approved rapid NGS solution,' highlights that the recently approved Oncomine Dx Express Test can provide companion diagnostic results from tissue samples within just 24 hours. This faster turnaround ensures that patients with high-risk thyroid cancer receive personalized treatment plans without substantial delays, thereby enhancing overall care coordination.
Key Players Profiled in the Thyroid Cancer Diagnostics Market
- F Hoffmann-La Roche AG
- Abbott Laboratories Inc.
- Thermo Fisher Scientific Inc.
- General Electric Co
- Siemens Healthcare GmbH
- Bio-Rad Laboratories Inc.
- Toshiba Corporation
- Koninklijke Philips NV
- Agilent Technologies Inc.
Report Scope
In this report, the Global Thyroid Cancer Diagnostics Market has been segmented into the following categories:Thyroid Cancer Diagnostics Market, by Type:
- Papillary Carcinoma
- Follicular Carcinoma
- Others
Thyroid Cancer Diagnostics Market, by Technique:
- Blood Test
- Imaging
- Biopsy
- Others
Thyroid Cancer Diagnostics Market, by End-use:
- Hospital Laboratories
- Cancer Diagnostic Centers
- Research Institutes
- Others
Thyroid Cancer Diagnostics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Thyroid Cancer Diagnostics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Thyroid Cancer Diagnostics market report include:- F Hoffmann-La Roche AG
- Abbott Laboratories Inc
- Thermo Fisher Scientific Inc
- General Electric Co
- Siemens Healthcare GmbH
- Bio-Rad Laboratories Inc
- Toshiba Corporation
- Koninklijke Philips NV
- Agilent Technologies Inc
Table Information
| Report Attribute | Details |
|---|---|
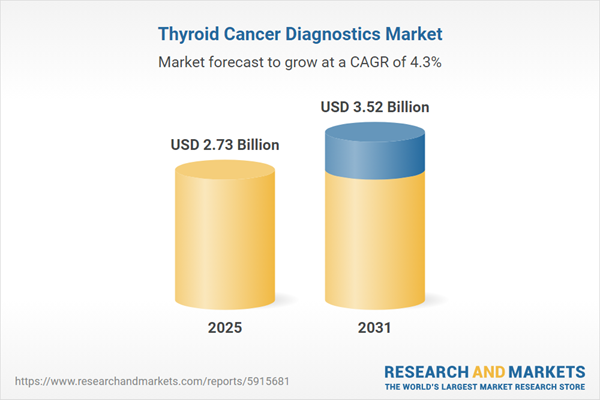
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 2.73 Billion |
| Forecasted Market Value ( USD | $ 3.52 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |