Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, market expansion faces significant impediments due to stringent regulatory hurdles related to the safety profiles of emerging therapies. Health authorities have established rigorous standards for novel candidates, particularly P2X3 antagonists, because of concerns regarding adverse effects like taste disturbances. This intense scrutiny requires extensive clinical validation and has caused delays in product approvals within key regions, acting as a major barrier to the rapid commercialization of new treatments and the overall growth of the global sector.
Market Drivers
The introduction of novel P2X3 receptor antagonists is a primary catalyst transforming the Global Cough Hypersensitivity Syndrome Treatment Market. These agents target P2X3 receptors on airway sensory neurons to block the ATP signaling pathway that drives the hypersensitive cough reflex, offering a highly specific mechanism compared to traditional central nervous system suppressants that addresses the physiological root of neuronal sensitization. Market confidence is being accelerated by the clinical validation of these candidates; for example, the American Journal of Respiratory and Critical Care Medicine reported in March 2025 that Phase IIb results for the P2X3 antagonist camlipixant showed a 34% placebo-adjusted reduction in 24-hour cough frequency. This targeted efficacy is a pivotal driver, promising better outcomes for patients who historically have had no effective options.Concurrently, the market is propelled by a strong clinical pipeline and increasing pharmaceutical R&D investment focused on diverse neurological targets. To address the heterogeneous nature of the condition, companies are exploring mechanisms beyond P2X3 inhibition, such as opioid receptor agonists. As noted by FirstWord Pharma in March 2025, the investigational therapy Haduvio achieved a statistically significant 57% placebo-adjusted reduction in cough frequency in the Phase IIa RIVER trial. This aggressive innovation is crucial given the substantial patient burden; a July 2025 study in ERJ Open Research identified that up to 27.9% of the chronic cough population suffers from possible refractory or unexplained chronic cough, underscoring a major industry shift toward precision medicine.
Market Challenges
The growth of the Global Cough Hypersensitivity Syndrome Treatment Market is significantly restricted by stringent regulatory hurdles concerning the safety profiles of emerging therapies. Health authorities have mandated rigorous standards for novel candidates, especially P2X3 antagonists, due to documented risks of adverse effects such as taste disturbances. This intense scrutiny necessitates extensive clinical validation, leading to prolonged development timelines and substantial delays in product approvals, which directly hinders the rapid commercialization of new treatments.These regulatory bottlenecks create a critical barrier to meeting the needs of patients who do not respond to standard care, thereby limiting the revenue potential for market participants. The magnitude of this missed opportunity is underscored by the prevalence of difficult-to-treat cases that remain unaddressed due to these delays. According to the European Respiratory Society in 2024, research indicated that up to 27.9% of individuals with chronic cough in assessed populations were classified as having possible refractory or unexplained chronic cough. Consequently, the inability to swiftly navigate these safety regulations stalls the introduction of vital neuromodulators, effectively capping the expansion of the global sector.
Market Trends
The integration of AI-driven acoustic cough monitoring is fundamentally reshaping the management of Cough Hypersensitivity Syndrome by shifting diagnosis and treatment efficacy tracking from subjective patient recall to objective, continuous data analysis. This technology not only improves diagnostic precision but also enables the delivery of digital therapeutics that directly address the behavioral components of the condition. The clinical utility of these platforms is becoming increasingly clear; in October 2025, Hyfe, Inc. reported that a pivotal study showed digital Behavioral Cough Suppression Therapy reduced average cough frequency by 41.8% in patients with refractory and unexplained chronic cough. Such advancements highlight a market trend where software-as-a-medical-device (SaMD) acts as an integral companion to pharmacological interventions, offering a non-invasive method to enhance patient quality of life.Simultaneously, the establishment of specialized chronic cough clinics is gaining momentum as a structural response to the disease's heterogeneity, facilitating multidisciplinary care models that include physiotherapy, speech therapy, and precise phenotyping. These dedicated centers are essential for stratifying patients who fail standard antitussive therapy and for fostering large-scale real-world evidence generation through organized registries.
This infrastructural expansion is exemplified by major pan-European initiatives; as detailed in the European Respiratory Journal Open Research in September 2025, the NEuroCOUGH Chronic Cough Registry was launched to recruit 2,500 patients across 13 European sites to standardize specialist care and improve the clinical trial infrastructure for emerging therapies. This trend signifies a move towards a more segmented and rigorous care pathway, ensuring that novel treatments are matched with the most suitable patient phenotypes.
Key Players Profiled in the Cough Hypersensitivity Syndrome Treatment Market
- Pfizer Inc.
- GlaxoSmithKline plc
- Boehringer Ingelheim GmbH.
- AstraZeneca plc
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG.
- Merck & Co. Inc.
- Vertex Pharmaceuticals Incorporated.
- Johnson & Johnson Consumer Inc.
Report Scope
In this report, the Global Cough Hypersensitivity Syndrome Treatment Market has been segmented into the following categories:Cough Hypersensitivity Syndrome Treatment Market, by Drug Class:
- Antitussive Agents
- Inhaled Corticosteroids
- Short Acting Beta-2 Agonists
- Anti-Cholinergics
- Antihistamines
- Proton Pump Inhibitors
- Others
Cough Hypersensitivity Syndrome Treatment Market, by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Cough Hypersensitivity Syndrome Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cough Hypersensitivity Syndrome Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cough Hypersensitivity Syndrome Treatment market report include:- Pfizer Inc.
- GlaxoSmithKline PLC.
- Boehringer Ingelheim GmbH.
- AstraZeneca PLC.
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG.
- Merck & Co. Inc.
- Vertex Pharmaceuticals Incorporated.
- Johnson & Johnson Consumer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
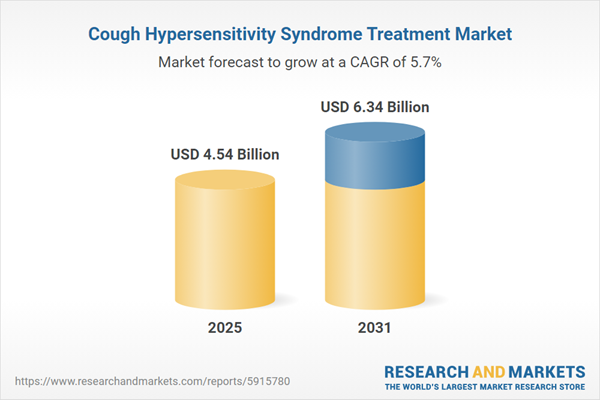
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 4.54 Billion |
| Forecasted Market Value ( USD | $ 6.34 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |