Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The market is witnessing significant growth due to the increasing prevalence of respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma, and acute respiratory distress syndrome (ARDS). The expanding applications of inhaled nitric oxide in neonatology, where it is employed to treat persistent pulmonary hypertension in newborns, contribute substantially to market expansion.
For instance, in the U.S., the Food and Drug Administration (FDA) enforces strict guidelines for the approval and use of inhaled nitric oxide systems, which include extensive clinical trials and post-market surveillance. The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) classifies nitric oxide as hazardous, requiring meticulous handling and disposal. These regulations are also applicable internationally, creating additional challenges for market participants operating globally.
The market is characterized by a surge in research and development activities, aiming to explore novel applications and enhance the efficacy of inhaled nitric oxide therapies. Pharmaceutical companies are actively engaged in clinical trials to assess the feasibility of inhaled nitric oxide in addressing conditions beyond its conventional use, such as in the treatment of COVID-19-related respiratory complications. This diversification of applications is fostering innovation and driving market growth.
Key Market Drivers
Rising Prevalence of Respiratory Disorders
The rising prevalence of respiratory disorders stands as a primary catalyst propelling the global inhaled nitric oxide (iNO) market to unprecedented heights. Over the past few years, there has been a noticeable surge in the incidence of respiratory conditions worldwide, creating an urgent demand for innovative and effective treatment modalities. Conditions such as acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and pulmonary hypertension have become more prevalent, necessitating advanced therapeutic interventions. For instance, the Centers for Disease Control and Prevention reported that in 2022, approximately 1 in every 10 infants born in the U.S. was preterm. Preterm birth, defined as delivering a baby before 37 weeks of pregnancy, presents substantial health risks to infants, including respiratory distress syndrome and persistent pulmonary hypertension.Inhaled nitric oxide has emerged as a crucial player in the healthcare sector, offering a promising solution for managing and improving outcomes in patients with respiratory disorders. Its vasodilatory effects and ability to enhance oxygenation make it particularly valuable in the treatment of conditions where respiratory function is compromised. The global healthcare landscape is witnessing a paradigm shift with an increasing focus on addressing respiratory health issues, and inhaled nitric oxide has positioned itself at the forefront of this transformative wave. For instance, according to April 2021 statistics from the Asthma and Allergy Foundation of America, approximately 20 million adults aged 18 and older in the United States suffer from asthma. This high prevalence of respiratory disorders in the country creates a steady demand for inhaled nitric oxide, contributing to the growth of the market in the United States.
Key Market Challenges
High Cost of Inhaled Nitric Oxide Treatment
The intricate processes involved in the production, storage, and delivery of inhaled nitric oxide contribute to the elevated overall treatment expenses. The cost-intensive nature of these procedures poses a challenge to the affordability of inhaled nitric oxide treatment, particularly in regions where healthcare budgets are constrained. Striking a delicate balance between ensuring profitability for manufacturers and affordability for healthcare systems is imperative for the sustained growth of the market.The high cost of inhaled nitric oxide treatment exacerbates global disparities in healthcare access. Regions with limited financial resources may find it challenging to integrate this therapeutic option into their healthcare systems, potentially leaving patients without access to a treatment that could significantly improve their respiratory conditions. Bridging this accessibility gap requires concerted efforts to develop pricing models that accommodate diverse economic landscapes.
Key Market Trends
Rise Of Telehealth and Remote Patient Monitoring
The rise of telehealth and remote patient monitoring is playing a pivotal role in boosting the Global Inhaled Nitric Oxide (iNO) Market. As healthcare continues to evolve, telehealth platforms and remote monitoring technologies have become integral components in the management of respiratory disorders, contributing significantly to the growth of inhaled nitric oxide therapies.Telehealth facilitates virtual consultations, allowing healthcare providers to remotely assess patients' respiratory conditions, discuss symptoms, and make informed decisions about treatment plans. In the context of inhaled nitric oxide, this trend enables healthcare professionals to monitor patients' responses to treatment in real-time, adjusting dosages or interventions as necessary. This remote monitoring capability not only enhances the efficiency of healthcare delivery but also ensures that patients receive timely and personalized care, particularly in the management of chronic respiratory conditions such as chronic obstructive pulmonary disease (COPD) or asthma.
Key Market Players
- Linde PLC
- Air Liquide Healthcare
- BOC Healthcare
- Matheson Tri-Gas Inc.
- Merck KGaA
- Mallinckrodt Pharmaceuticals (Novoteris)
- Nu-Med Plus Inc.
- Perma Pure LLC
- Praxair Distribution Inc.
- HALMA PLC
Report Scope:
In this report, the Global Inhaled Nitric Oxide Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Inhaled Nitric Oxide Market, By Product Type:
- Gas
- Delivery Systems
Inhaled Nitric Oxide Market, By Application:
- Neonatal Respiratory Treatment
- Asthma and COPD
- Acute Respiratory Distress Syndrome
- Malaria Treatment
- Tuberculosis Treatment
- Other Applications
Inhaled Nitric Oxide Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Egypt
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Inhaled Nitric Oxide Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Linde PLC
- Air Liquide Healthcare
- BOC Healthcare
- Matheson Tri-Gas Inc.
- Merck KGaA
- Mallinckrodt Pharmaceuticals (Novoteris)
- Nu-Med Plus Inc.
- Perma Pure LLC
- Praxair Distribution Inc.
- HALMA PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
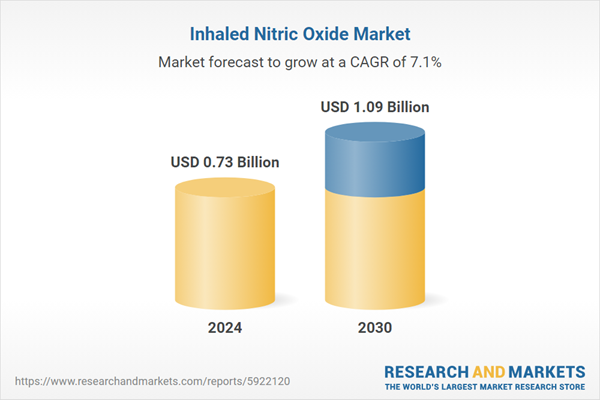
| Estimated Market Value ( USD | $ 0.73 Billion |
| Forecasted Market Value ( USD | $ 1.09 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |