Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the industry faces significant hurdles related to the complex biology of disease progression. A major obstacle impeding market growth is the high variability of resistance mechanisms found within the tumor microenvironment. This heterogeneity complicates the ability of pharmaceutical developers to identify universal biomarkers and create effective combination protocols.
Market Drivers
A primary accelerator for the market is the expansion of the addressable patient pool resulting from increased frontline immunotherapy adoption. As immune checkpoint inhibitors become the standard of care for earlier stages of various malignancies, the number of patients eventually progressing on or after these therapies rises, creating a distinct population in need of salvage interventions and compelling developers to prioritize agents that overcome acquired resistance. For instance, Merck’s 'First Quarter 2024 Financial Results' in April 2024 reported a 20% increase in worldwide Keytruda sales to $6.9 billion, illustrating the massive scale of frontline adoption that feeds the pipeline of patients potentially eligible for subsequent lines of therapy.Simultaneously, a surge in strategic alliances and funding for next-generation immuno-oncology research is driving a robust pipeline of combination therapies. Developers are actively acquiring assets such as T-cell engagers and antibody-drug conjugates to bypass the immunosuppressive tumor microenvironment that renders initial checkpoint blockade ineffective. Exemplifying this trend, Merck announced in January 2024 that it invested approximately $680 million to acquire Harpoon Therapeutics to advance a portfolio of T-cell engagers targeting immunotherapy-resistant solid tumors. This investment activity aligns with continued clinical validation of new modalities, as evidenced by Iovance Biotherapeutics’ February 2024 press release stating that the cell therapy Amtagvi achieved an objective response rate of 31.5% in metastatic melanoma patients previously treated with a PD-1 blocking antibody.
Market Challenges
The extensive variability of resistance mechanisms within the tumor microenvironment presents a formidable barrier to the growth of the Global Checkpoint Inhibitor Refractory Cancer Market. This biological heterogeneity implies that a treatment protocol effective for one patient often fails in another due to differing immune evasion pathways, thereby complicating the pharmaceutical development process. Consequently, researchers struggle to validate universal biomarkers that can reliably predict patient response to salvage therapies, forcing developers to conduct larger, costlier, and longer clinical trials to prove statistical significance, which frequently delays regulatory approvals and commercial product launches.Furthermore, the inability to easily categorize resistance types restricts the addressable patient pool for new agents and limits the scalability of emerging drugs. According to the Cancer Research Institute in 2024, while approximately 45 percent of newly diagnosed cancer patients were eligible for immunotherapy, the intricate nature of tumor resistance continues to exclude the majority from effective treatment options. This limitation hampers market revenue potential, as companies cannot readily scale therapies across broader refractory populations without the aid of precise, unified biological targets.
Market Trends
The advancement of personalized mRNA neoantigen vaccines in combination settings is emerging as a critical strategy to restore immune recognition in resistant malignancies. Unlike standard immunotherapies, these vaccines encode specific neoantigens from a patient’s tumor profile, priming T-cells to attack cells that have evaded prior blockade, an approach gaining momentum as developers pair vaccines with PD-1 inhibitors to enhance durability. According to a Merck press release in June 2024, data showed that the combination of mRNA-4157 (V940) and Keytruda reduced the risk of recurrence or death by 49% compared to pembrolizumab alone in patients with high-risk stage III/IV melanoma.Concurrently, the strategic integration of Antibody-Drug Conjugates (ADCs) into post-immunotherapy regimens is reshaping the landscape for patients progressing after checkpoint inhibition. By delivering cytotoxic payloads directly to antigen-expressing tumor cells, ADCs provide a mechanism to bypass the immunosuppressive microenvironment responsible for checkpoint resistance, making them a preferred salvage therapy in solid tumors where continued immunotherapy is ineffective. Highlighting this shift, Gilead Sciences reported in its 'Second Quarter 2024 Financial Results' in August 2024 that sales of the ADC Trodelvy rose 23% to $320 million, primarily driven by demand in second-line metastatic triple-negative breast cancer where patients frequently require effective interventions after exhausting initial immune-based options.
Key Players Profiled in the Checkpoint Inhibitor Refractory Cancer Market
- Bristol-Myers Squibb Company.
- AstraZeneca plc
- Merck KGaA
- F. Hoffmann-La Roche Ltd.
- Regeneron Pharmaceuticals Inc.
- Pfizer Inc.
- Janssen Global Services, LLC
- 4SC AG
- Mirati Therapeutics, Inc.
- Ascentage Pharma
Report Scope
In this report, the Global Checkpoint Inhibitor Refractory Cancer Market has been segmented into the following categories:Checkpoint Inhibitor Refractory Cancer Market, by Type:
- PD-1 Inhibitor
- PD-L1 Inhibitor
- Others
Checkpoint Inhibitor Refractory Cancer Market, by Application:
- Lung Cancer
- Bladder Cancer
- Melanoma
- Hodgkin Lymphoma
- Others
Checkpoint Inhibitor Refractory Cancer Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Checkpoint Inhibitor Refractory Cancer Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Checkpoint Inhibitor Refractory Cancer market report include:- Bristol-Myers Squibb Company.
- AstraZeneca PLC.
- Merck KGaA
- F. Hoffmann-La Roche Ltd
- Regeneron Pharmaceuticals Inc
- Pfizer Inc
- Janssen Global Services, LLC
- 4SC AG
- Mirati Therapeutics, Inc.
- Ascentage Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
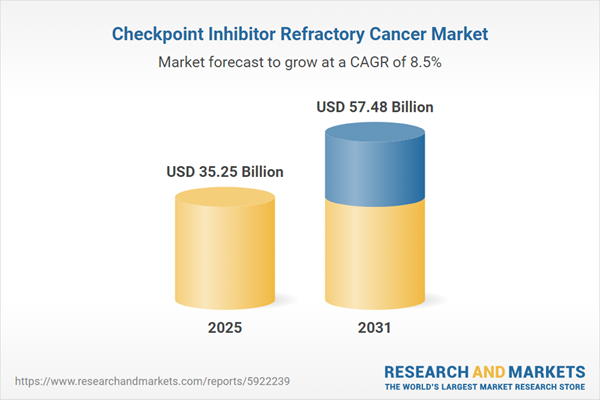
| Estimated Market Value ( USD | $ 35.25 Billion |
| Forecasted Market Value ( USD | $ 57.48 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |